Phylum Rotifera |
||
AuthorsEditor |
Rotifers are a diverse group of microscopic pseudocoelomate animals, which are nearly ubiquitous in fresh and sea water. Rotifers also occur on sphagnum in marshes and on moss in moist forests. This phylum includes about 2000 known species (Segers, 2007). Most rotifers are free swimming, but some are sessile. A small group of rotifers lives on other animals or plants. Only a few dozen species can reach 1–2 mm in body size, the remaining species are much smaller. The average body length of a rotifer is 0.1–0.5 mm. The colonies of rotifers can reach 3-4 mm in diameter. Rotifers are characterized by highly specialized tissues and a fixed number of cells, or eutely. Another characteristic feature of rotifers is a specialized wheel organ called the corona. The corona is a ciliary organ provided with intrinsic musculature, which is used for prey capture and locomotion. The beating of cilia of the corona resembles the flickering of wheel spokes, hence the Latin name of the phylum, Rotifera, i.e. “bearing wheels”. The body of rotifers contains internal organs, including sclerotized jaws called the mastax. At the posterior end of the body is a muscular appendage, the foot, which rotifers use to crawl or to attach to the substrate. The tip of the foot often bears a pair of processes called “toes”. Rotifers can be “naked” (illoricate) (Fig. 1A) or their body can be surrounded by a protective casing (lorica) (Fig. 1B). Rotifers are classified into 4 groups by the way they move: swimming-crawling, swimming, crawling and sessile. |
Contents


Fig. 1. General view of rotifers: А – illoricate rotifer Asplanchna priodonta, B – loricate rotifer Brachionus sp. Differential interference contrast (DIC). Photo by E.A. Kotikova

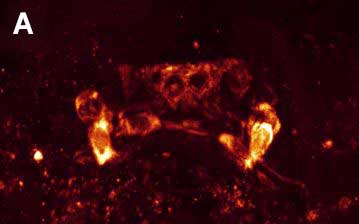
Fig. 3. Double ICC staining of the nervous system of the rotifer Platyias patulus. FMRFamide-IR (red) and 5-HT-IR (green). Photo by E.A. Kotikova
|
Classification of rotifers |
Rotifers are dioecious animals. The reproductive system of females is an undivided (Monogononta) or bilobular (Digononta) ovary. At the end of the 19th century, this difference was used to divide all rotifers into two groups (Wesenberg-Lund, 1899). This classification has persisted to the present. Russian scientists attempted to develop a new system based on the morpho-functional approach in studying key structures of rotifers, their corona and mastax (Kutikova, 1970; Markevich, 1991). Unfortunately, this new system has gained no traction outside Russia and remains largely unknown. In recent years, molecular phylogenetic studies have significantly revised the phylogeny of the bilateral animals (Bilateria), but they have not clarified entirely the phylogenetic position of rotifers. The name Syndermata was proposed for the group uniting rotifers and Acanthocephala (proboscis worms); this name emphasizes a synapomorphy shared by rotifers and acanthocephalans: a unique syncytial epidermis with an intracellular skeletal lamina (Ahlrichs, 1997; Barnes et al., 2001). Nevertheless, the phylogenetic relationships within Syndermata remain unknown. A recent study by Fontaneto and Jondelius (2011) has examined over a thousand sequences of COI and showed the monophyly of the clades of Acanthocephala and rotifers: Bdelloidea, Monogononta and Seisonidea. The interrelationships between these clades remain unclear, except that Acanthocephala and Bdelloidea were clearly shown to be sister groups. |
|
Nervous system |
The first significant advance in studying all organ systems of rotifers including the nervous system was made by A. Remane (1929-32), who compiled and analyzed the results of classical histological studies. The ultrastructure of rotifers was later studied by electron microscopy revealing a number of new aspects in the architecture of their nervous system. Brain neurons of rotifers were shown to contain at least 4 types of synaptic vesicles (Villeneuve, Clément, 1971; Clément, 1977; Wurdak et al., 1983). The smallest of these vesicles had electron lucent core and were assumed to contain acetylcholine; the presence of acetylcholine in these vesicles was later confirmed by biochemical and histochemical studies without neural mapping (Nogrady, Alai, 1983). Other brain neurons were shown to have vesicles of larger size with an electron dense core; these vesicles could contain catecholamines (CA) or serotonin. A combination of three different bio- and histochemical techniques has identified catecholamines in the nervous system of rotifers, but once again failed to show the arrangement of these neurons in the nervous system (Keshmirian, Nogrady, 1987, 1988). In the early 1990s, the Laboratory of Evolutionary Morphology of the Zoological Institute RAS has begun studying the distribution of catecholamines in the nervous system of rotifers. The main technique of these studies was Kabotyansky’s (1985) modification of the histochemical method of glyoxylic acid-induced fluorescence (GIF) with a five-fold increase in concentration of glyoxylic acid in the incubation solution. The studies were conducted on 12 species of rotifers, of which 2 were from one family of Digononta and 10 were from 7 distantly-related families of Monogononta (Kotikova, 1995; 1998a, b). The major differences in the architecture of the CA-ergic nervous system were observed in the arrangement of brain neurons (Fig. 2).
Fig. 2. Diagram of the arrangement of CA-ergic neurons (black) in the brains of rotifers. A – Philodina sp. B – Manfredium eudactylotum. C – Platyias quadricornis. D – Asplanchna herricki. E – Euchlanis dilatata. F – Notommata sp. G – Lecane arquata. H – Brachionus quadridentatus. I – Dicranophorus forcipatus.
Three grades of increasing complexity have been identified in the arrangement of brain neurons: Х-shaped (with three or four rows), arcuate (arch-shaped) with a wide or narrow curve and ring-shaped. Brain neurons in Philodina and Rotaria (Fig. 2А) were stretched in the longitudinal direction in three or four rows to form Х-shaped structures (Kotikova, 1995). In rotifers with an arc-shaped brain, the wide curve was present in Platyias (Fig. 2С) and the narrow curve in Euchlanis (Fig. 2E). The transition from the arc-shaped to the ring-shaped brain has probably occurred in two successive steps. During the first step, a small curvature has been formed in the brain; this brain type is exemplified by a half-ring brain of Lecane (Fig. 2G). The second step has resulted in a closed elliptical brain complex of Brachionus and Dicranophorus (Fig. 2H, I). In all cases, the ventrolateral cords arise from the lateral neurons of the brain. In addition to the brain neurons, some nerve cells are situated along the cords and also in the area of the head and mastax (pharynx of rotifers with a specialized masticatory apparatus). The brain neurons account for 20% of the total number of cells in rotifers (Martini, 1912). Three-dimensional reconstruction using a computer program has demonstrated a clear bilateral symmetry in the arrangement of 200 brain neurons in Asplanchna brightwelli (Ware, Lopresti, 1975). The total number of CA-ergic brain neurons in our material is small (6-11), but this number is constant for each species and accounts for 3-6% of the total number of neurons in the brain complex. The only exception is Notommata; Hochberg (2007) has revealed 40 cell bodies containing FMRFamide or 5-HT in the brain of Notommata copeus, and in Notommata sp. there are four CA-ergic brain neurons (Kotikova, 1998a), which is 22% of the total number of nerve cells. The presence of synaptic vesicles with as yet unknown chemical composition has provided the impetus for starting immunocytochemical studies of the nervous system in rotifers and for identifying other neurotransmitters. |
|
Immunocytochemical study of the nervous system |
Immunocytochemical (ICC) studies using antibodies against polypeptide FMRFamide and the monoamine serotonin (5-HT) were conducted on three species of rotifers from the phylogenetically advanced order Ploima: the loricate swimming-crawling rotifers Platyias patulus and Euchlanis dilatata and the illoricate swimming rotifer Asplanchna herricki. In Platyias patulus (Fig. 3), eight cerebral FMRFamide-IR neurons are arranged in 2 rows to form an arch-shaped pattern: a pair of small bipolar neurons are located in the anterio-dorsal half-ring, a pair of unipolar neurons in the region of the corona and 2 pairs of neurons along the ventro-lateral longitudinal cords (Kotikova et al. 2005). The brain contains six 5-HT IR neurons arranged in three rows in an Х-shaped pattern, 2 pairs of bipolar neurons at the anterio-dorsal half-ring and a pair of bipolar neurons along the longitudinal cords (Fig. 3). The neurites of FMRFamide-IR and 5-HT-IR neurons in the longitudinal cords run parallel to each other, while the cell bodies of the same IR are positioned independently of each other in the brain and along the cords. Euchlanis dilatata has 6 pairs of FMRFamide-IR neurons (Kotikova et al., 2005): two pairs lie in an arc-shaped arrangement, two other pairs are positioned along the nerve cords, and another pair of multipolar neurons is located in the region of the corona (Fig. 4). Two pairs of 5-HT-IR neurons located in the lateral parts of the brain are arranged in an arch-shaped pattern, and 2 pairs of bipolar neurons are located along the cords. In Asplanchna herricki (Kotikova et al., 2005), fourteen cerebral FMRFamide-IR neurons form a ring-shaped structure: 6 have a hexagonal shape (ventral side of the brain), the other 8 lie on the dorsal side and are tear-shaped or spherical. Another pair of bipolar neurons is located on the nerve cords (Fig. 5А). Two pairs of 5-HT-IR neurons are located in the arc-shaped neuropil: one pair is median, these neurons are smaller (3.5 µm) and more brightly stained, the other pair lies more laterally, these neurons are larger (5 µm) and less intensively stained. Two pairs of neurons, proximal and distal, are associated with the ventrolateral cords (Fig. 5B). In all studied species, double ICC staining has demonstrated an independent arrangement of neurons 2 to 10 µm in size. Neurites of different immunoreactivity are always oriented parallel to each other, and the FMRFamide IR neurites run underneath the 5-HT IR neurites. No colocalization of neurotransmitters was found. One year after the publication of our study, R. Hochberg has published a paper describing the distribution of serotonin-ergic elements in the nervous system of 2 planktonic rotifers Conochilus coenobasis and C. dossuarius (Hochberg, 2006). In these species, the 5-HT-ergic nervous system consists of a dorsal cerebral ganglion, 4 pairs of cell bodies grouped in clusters and a pair of longitudinal nerve cords connected with each other in the foot. The second paper of the same author that has been published the next year describes in detail FMRFamide- and 5-HT IR regions in the nervous system of the benthic rotifer Notommata copeus (Hochberg, 2007). FMRFamide and 5-HT were found in the brain and in a pair of longitudinal cords. The cerebral ganglion includes thirty FMRFamide- and ten 5-HT IR cell bodies. Peptide-ergic cell bodies lying on the periphery of the brain show only weak fluorescence, while some of the central cell bodies are more brightly stained. The distribution of 5-HT IR elements was also described in the bdelloid rotifer Macrotrachela quadricornifera (Leasi et al., 2009). A similar arrangement of CA-ergic, FMRFamide IR and 5-HT IR brain neurons in the species that belong to three different families of the same order Ploima suggests an independent and parallel evolution of these structures in phylogenetically distant rotifers. Only one type of arrangement of the cerebral structures (X-shaped) was described in lower Bdelloida, whereas higher rotifers have all three types: Х-shaped (with 2-3 rows), arcuate and ring-shaped. |
|
Recommended literature |
|
|