
 |
Caspian Sea Biodiversity Project under umbrella of Caspian Sea Environment Program |
| Biodiversity Report | Chronology | Sources | Collections | Check-Lists | Contacts |
by N. Aladin, I. Plotnikov, A. Bolshov (head of biodiversity thematic center of Caspian Environment Program, Atyrau, Kazakhstan), A. Pichugin (Tethys Consultants)
The Caspian Sea is the largest lake, both by its area and volume. The watershed of this large water body is approximately 3.5m km2 that makes more than 10 % of all drainless areas on the earth. The Caspian has a lot of unusual features. Its level is lower than the mean sea level (M.S.L.). In the 20th century it has been fluctuating within the range of almost four meters, approximately from -25 ì in the beginning of the century to -29 ì in 1977. In the end of the 20th century, the sea levels unexpectedly soared, and in the beginning of the 21st century began to drop again. The Caspian is not a freshwater lake. Its waters are rather brackish; three times less concentrated than in the World Ocean. As well as all land-locked lakes, the water balance of the Caspian hinges on precipitation, river and ground water runoffs, and evaporation, which are directly linked to atmospheric circulation.
The Caspian Sea has also unusual origins. It is a remnant of the ancient northern gulf of the Thetis Ocean, which used to connect the Atlantic and the Pacific Oceans. The Caspian can be compared to Australia because it was separated from the World Ocean a long ago, as Australia was from other continents. Many rare animals and plants, which are called living fossils, have survived both in the Caspian and Australia due to the isolation.
The Caspian is not a homogeneous lake. Three different water bodies are united within its borders, each of them has its own physical conditions and biological diversity.
Of these, the first water body is its northern area. The second is its middle and southern areas. The third is the shallow gulf Kara-Bogaz-Gol, located at eastern coast of the Caspian. Of these three water bodies, the second one is the largest. It contains some 99 % of the total volume of the sea and occupies two thirds of its surface. Many researchers consider this part of the Caspian Sea as the real Caspian. The water salinity is almost stable here fluctuating within 12-13 gr/l. This is the normal salinity of the Caspian. This part of the sea is inhabited by the greatest number of unique Caspian species, which descend from ancient inhabitants of the Thetis. This is the deepest part of the sea, which in the southern basin exceeds 1 km. This part of the Caspian has never completely dried up, independently of climate changes that occurred in past geological epochs. A sufficient amount of water has always remained here, consequently preserving the life in it, though the increased water salinity usually resulted in reduction of the number of inhabitants in such regressive phases.
The area and volume of the northern water body is smaller. It contains about 1 % of the volume of the Caspian, and its area amounts to one third of the total area of the Caspian. Its water salinity is not stable and, as a rule, is lower than that of the Middle Caspian by a factor of 2 or 3 due to river runoffs. Namely, the Volga meets the Caspian Sea here, and this river provides about 80 % of the total river runoff. Many unique Caspian species rarely occur in this part of the Caspian because of low water salinity, however some of them run to this area to spawn. Many freshwater organisms range only here and never occur in the Middle and Southern Caspian, where the water salinity is too high for them. This part of the Caspian is shallow and maximal depths never surpass 10 m. Due to shallowness, this area repeatedly dried up in past geological epochs, and its inhabitants either perished, or lived in deltas and estuarine reservoirs of Caspian rivers through unfavorable periods.
The gulf Kara-Bogaz-Gol is the smallest by its area and volume. Allegedly, it is the third water body within the Caspian. Though its area makes only 3 % of the area of the whole Caspian, and its volume is significantly small, this reservoir deserves special attention. Its surface is below the level of the Caspian by several meters and a lot of water constantly drains into it. This water quickly evaporates at shoals of the gulf and the water salinity is extremely high there: ten and even twenty times the water salinity of the Middle Caspian. Inhabitants of the Caspian Sea don’t live here because of the high salinity. The gulf is inhabited only by salt-loving organisms. This area repeatedly changed its outlines during climate changes of past geological epochs. The gulf used to become either very vast, or completely dried.
The above information shows that it is difficult to contemplate about general biological diversity of the Caspian Sea. The distinct differences in physical conditions of the three water bodies, considered above, result in distinct differences in biodiversity. The northern water body has the richest fauna and flora. It’s ensued by the Middle and Southern Caspian, and the lowest biodiversity is observed in the gulf Kara-Bogaz-Gol. However, the overall picture of life distribution in the Caspian won’t be full if we do not take into consideration inhabitants of the deltas of the Caspian rivers. Various plants and animals inhabit estuaries of these rivers. The real biodiversity of the Caspian Sea is made up of that of all water bodies, including deltas and their wetlands.
The present book addresses the biological diversity of the Caspian Sea. We tried to inform readers of the surprising world of inhabitants of the Caspian, we described the history of studies of these inhabitants and threats posed by human activities.
This book describes in-depth history of the development of the Caspian Sea, the physical environment of the water bodies and major habitats and species ranging there. Special attention will be paid to commercial species and also to both rare and endangered animals and plants. Particular attention is drawn to invaders of the Caspian Sea. The book shows that practically all inhabitants of the Caspian can be called invaders. However, nowadays they are considered to be indigenous, since most of them have invaded the Caspian millions years ago.
One particular chapter addresses existing threats to biological diversity of the Caspian Sea. Negative impact of the following factors on inhabitants of the Caspian were analyzed in details: river flow control, poaching, water level fluctuations, pollution, exotic species and climate changes. This chapter concludes that oil pollution and recent invasion of the comb-jelly can seriously damage the biological diversity of the sea. It was shown that these effects can be both long-term (chronic) and acute (short-term).
The penultimate chapter is dedicated to the history of studies of the biological diversity of the Caspian. This chapter demonstrates the great input of researchers of all Caspian littoral countries. In the same time, it is emphasized that researchers from the Russian Empire and the former Soviet Union made the major input. The chapter concludes that the breakup of the USSR and emergence of new Caspian littoral states, because of difficult economic problems they face, have negatively affected the biological diversity of the Caspian Sea. Since that the poaching has intensified, failures in operations of hatcheries have become more frequent, and the number of accidents at oil and other offshore technical facilities has increased because of deteriorated and obsolete equipment. There is a fear that the Caspian Sea, famous for its sturgeon stocks amounting to 90 % the world stocks, can soon loose these highly valuable commercial stocks. An analysis of available data on biological diversity of the Caspian and reasons of the deficit is given in this chapter. Information on available databases and their management is provided.
The final chapter is dedicated to conclusions and proposals at a political level.
The word biodiversity is made of two words. A Latin word bios meaning – the life and English - diversity. This new word has appeared in our language rather recently. A UN Conference dedicated to the environment and development was held in Rio de Janeiro in 1992. The conference has approved the Biodiversity Convention. This event was broadly covered in the mass media around the world including our country. In that period a shortened new word emerged - biodiversity. Following that use of the term has become widespread.
The biodiversity is often subdivided into three hierarchical levels. The first level refers to genetic biodiversity or diversity of one species. Individual families, populations, geographical races of one species of an animal or a plant can serve a good example of such a level of biodiversity. The second level refers to specific biodiversity or diversity of species. The third level refers to ecosystem biodiversity or diversity of ecosystems.
The definition of biodiversity was proposed at the mentioned Conference of 1992: "variability of living organisms of various origins, including, inter alia, land, marine and other aquatic ecosystems and ecological complexes, which they belong to; this includes diversity of species and ecosystems ". The term “biodiversity” is most frequently used for assessment of the number of species found at a certain area or in a water body. So, for example, if there are many animal and plant species inhabiting a lake, then the lake is said to have high biodiversity, if there aren’t many species then low biodiversity. As it was already indicated above, the biodiversity can be analyzed at an ecosystem level. The Rio de Janeiro Conference gave the following definition to an ecosystem: "a dynamic complex of communities of plants, animals and microorganisms, and their lifeless habitats, interacting as a functional unit". A living organism cannot be separated from its habitat, therefore the study of the biodiversity assumes studying of both animals and plants, and the surrounding environment.
Therefore, we shall briefly consider all three levels of biodiversity relating to the Caspian Sea and its inhabitants. Let's begin from the highest ecosystem level. A large number inter-subordinate and interconnected ecosystems coexists in the Caspian. As in any other water body, it is possible to single out an ecosystem of a water stratum consisting of bacterioplankton, protozoan plankton, phytoplankton, zooplankton and nekton in the Caspian, as well as a bottom ecosystem consisting of benthic bacteria and protozoa, phytobenthos and zoobenthos. Ecosystems of open waters (deep-water and shelf) and coastal shoals as well as ecosystems of macrophyte thickets are easily distinguishable. Given different salinity in different parts of the Caspian, it is quite easy to mark out freshwater, oligohaline, mesohaline and hyperhaline ecosystems. Freshwater ecosystems are formed in deltas and estuaries of the Caspian influents. Oligohaline ecosystems are widespread on shoals of the Northern Caspian, where the water salinity ranges from 0.5 up to 5 gr/l. Almost the entire waters of northern part can be regarded as an oligohaline ecosystem. The waters of the Middle and Southern Caspian can be referred to as a mesohaline ecosystem. The average water salinity here is 12-13 gr/l. A typical hyperhaline ecosystem exists in the gulf Kara-Bogaz-Gol, where the water salinity is higher than 40 gr/l.
The above partial list of Caspian ecosystems shows high ecosystem biodiversity. It is linked to high diversity of physical parameters of the aquatic environment in the Caspian. The diversity of habitats encourages diversification of aquatic ecosystems.
Let's now briefly look at the biological diversity at a species level. At this level the biodiversity of the sea is also very high. This is due to complicated and long history of formation of the Caspian fauna and flora. Nowadays, the Caspian is inhabited by descendants of many ancient organisms, whose ancestors had penetrated into it some millions years ago. These are species originating from the disappeared Thetis Ocean, from arctic marine and freshwater reservoirs of the pre-glacial and the glacial periods, and also rather recent Atlantic and freshwater invaders. Due to this, the biological diversity of the Caspian Sea at a specific level is so high. By the number of animal and plant species this huge continental reservoir is one of the richest in the world, and by the number of living fossils, it is, obviously, incomparable.
The genetic biodiversity of Caspian hydrobionts is also high. However, in certain cases, it is lower, than it was believed earlier, prior to the inception large-scaled researches using genetic and molecular-biological methods. Certain inhabitants of the Caspian fauna and flora have so clear genetic differences in phenotypes of different populations that some zoologists and botanists even mistakenly cast these populations as independent species. We would like to cite only a few examples from many known. For a long time, many zoologists regarded a number of fishes and molluscs, which occur both in the Caspian and in neighboring reservoirs, as independent species. They even have developed special morphological diagnostic signs to distinguish these species. However, genetic researches showed that these organisms represented geographical races, or, at the most, subspecies, but not different species. In the same time, these researches have demonstrated that genetic differences between investigated hydrobionts really exist, however, they have convincingly proved that the extent of such differences is obviously insufficient for casting these species as independent species.
Caspian plankton crustaceans represent another clear example. Some of them are capable of parthenogenetic (without fertilization) reproduction during a warm season, and then they switch to sexual reproduction during a cold season, which results in production of latent (dormant) eggs. These eggs sink and wait out a cold period at the bottom. In spring, during a warm period, parthenogenetic females hatch out from these eggs. The water in southern part of the Caspian, at the Iranian coast, even in wintertime, is very warm and never lowers below 8-100C. Hence, these crustaceans can reproduce parthenogenetically round a year, producing numerous clones. Often, these clones were mistakenly described as an independent species. However, these "species" perished after special molecular-biological and genetic researches.
Why is it so important to study and to preserve biodiversity? The point is that the ambient living world is a result of the long-term biological evolution. For a long time, all levels of biodiversity (genetic, specific and ecosystem) formed and interacted naturally without human involvement. However, the development of the human civilization resulted in strong anthropogenic impact, which interferes with the natural course of events. The humankind has become a powerful external factor destabilizing the processes of the biosphere. Even ancient people started adversely affecting our planet, however such impacts had a local bearing. Many scientists believe that as far back as in ancient time the man destroyed several species of animals by hunting and many natural land ecosystems by ranging livestock and farming. Of course, before the humankind appeared on the earth, many herbivorous animals had been destroying the vegetative cover. However, when man tamed animals and started breeding them and artificially maintaining their high population on limited rangeland, then the damage to the vegetative cover increased by far. As to the farming, it almost completely destroys natural land ecosystems replacing them with artificial fields of cultivated plants. Human activities can inflict similar great damage to water bodies. Due to rapacious and unregulated fishing, often carried out during spawning, many valuable species are vanishing. Complete extinction of the Steller’s sea cow is a paradigm. This large marine mammal was simply hunted to the extinction. The forestlands were almost completely felled in many western European countries, where they existed before man came. Some researchers (Letolle, Mainguet, 1996) even suppose that many modern deserts had appeared rather due to inept activities of ancient people than because of climate changes.
In the end of the 20th century, the extent of human impacts on the biodiversity at all three levels ripened from local into global. New, large-scale negative impacts have appeared in addition to hunting, fishing, livestock breeding, farming and tree felling. First of all, we mean the industry. Bar the needed products manufactured by the industy, it has also discharged a great amount of pollutants into air, water and soil, inflicting irretrievable damage to the biodiversity. Industrial methods are now also employed in agriculture. A plethora of artificial chemicals are now utilized in agriculture to fertilize cultivated plants and combat weeds and pests. These substances seep into the ambient environment, damaging biological diversity. In arid regions, development of agriculture required large-scale artificial irrigation, thus leading to fast salinization and depletion of water resources in certain regions. The environmental disaster of the Aral Sea, brought by these reasons, is now notorious. The nuclear energy was discovered in the same century and now it is used both for peaceful and military purposes. It has also had a strong bearing on the biodiversity of our planet. Negative consequences of nuclear testing and bombardments and of the Chernobyl nuclear power plant disaster can be tracked thus far.
The above examples clearly show why it is necessary to study and to preserve the biodiversity. The mankind merely cannot exist without living organisms, since except for being a source of food and raw materials, they also supply our planet with air, which all living creatures breathe with. Of course, it is possible to live without species, which have been already destroyed by humans; however, our life becomes poorer without them. Today we are on the brink, where we need to stop. This was the rationale to held the above conference in Rio de Janeiro, which called the mankind to change its mind while it is not too late. Ecosystems consisting of genetically and taxonomically diverse components are better adapting to changes happening under the influence of natural or anthropogenic impacts. When there is high biodiversity, it is easier for the evolution to find perspective populations, which will gain further development and will save the life on our planet.
A UN Conference on Ecology and Development was held in Rio de Janeiro in 1992 with participation of more than 150 countries. The conference resulted in the establishment of the Convention on biological diversity. This document determines the biological diversity as "diversity of living organisms of all habitats, including land, marine and other aquatic ecosystems and ecological complexes, which they belong to; this definition includes the diversity of species and ecosystems".
Mostly, the term “biodiversity” is used to describe the number of species at a certain area (Gray, 1997). The biodiversity can also be considered at levels distinct from taxonomic organization, for example, at the level of a community or / and an ecosystem (Gray, 1997).
The convention on biodiversity determines the ecosystem as "a dynamic complex of communities of plants, animals and microorganisms, and their lifeless habitats, interacting as a functional unit ". The following sections of this document will consider the issues of specific diversity, diversity of communities or / and ecosystems and marine landscape or / and diversity habitats.
The convention contains 42 articles, which include specific obligations of each party and policy, resulting from such obligations. Article 6 encompasses the broadest range of issues with an emphasis on the need to:
The loss of habitats and their modification are the main factors causing current reduction of world biodiversity (WCMC 1992), therefore its momentous to conduct monitoring of present extension of changes of habitats and living conditions.
The goals of Article 1 of the Convention on Biological diversity include:
"Preservation of biological diversity sustainable use of its components, and joint and fair utilisation of genetic resources".
Therefore, the convention tends to designate adverse effects of human activities on the environment, ensuring that each party creates a system of protected areas for preservation of the biodiversity.
It is required to collect necessary information to assess efficiency of the biodiversity preservation strategy. Article 7 of the Convention on biodiversity describes methods to reach these goals, which include identification of important components of biological diversity and appropriate collection of specimens of these components, paying special attention to those components, which require urgent measures for their preservation.
Appendix I to Article 7 specifies groups, viz. ecosystems and habitats, requiring attention:
It is necessary to identify processes or mechanisms, which may unfavorably influence the preservation and sustainable use of biodiversity, and to trace their influence by means of taking samples and specimens.
It is necessary to establish and maintain databases, for storage of data obtained during identification and monitoring, in correspondence with the above.
It assumes that it is required to receive considerable amount of information in many fields to satisfactorily develop a biodiversity strategy and to meet requirements of the article.
Article 8 is one of the biggest articles of the Convention. It contains major obligations for preservation of biodiversity. This article indicates the need to establish a network of protected areas and to develop guidelines for selection, establishment and management of protected territories.
Article 8 also specifies the need to regulate or manage biological resources, which are important for the preservation of biodiversity to ensure their longevity. This includes strengthening ecosystem protection and environmentally sound, sustainable development of the regions located next to protected territories.
In addition to the above, Article 7 also encourages regulation of programs aimed at restocking and rehabilitation of rare and endangered species both in situ and ex situ.
The most effective way of preservation of biodiversity is to prevent deterioration of plant and animal habitats. So-called "hotspots" are often identified for this purpose. These are areas, for example, with an unusually high level of pollution and where the biodiversity is under the threat of loss. In addition to this, it is also practiced to identify especially protected areas or "points of special attention ". These territories are characterized by very high biodiversity, which should be carefully protected.
The above approach is suitable for the Caspian Sea. The Caspian littoral states should be aware of their "hotspots" and "points of special attention" to preserve the biodiversity of the sea. Appropriate organizations of these countries should undertake emergency actions to preserve as much biological diversity as possible in their area of responsibility. If any species and its habitat is located in a shared area of responsibility of several Caspian littoral countries, then these countries should develop joint coordinated transboundary activities for the preservation of such an organism and biotype. It is also necessary to develop as quick as possible, appropriate programs for rehabilitation and restoration of already destroyed by man habitats of vanishing threatened species. It is also necessary to pay special attention to commercial species. These species need protection from poachers and overfishing, through development of corresponding legal instruments. In regards to these species, it is also necessary to implement a restocking programme to keep up sufficient population for commercial use.
In correspondence with the provisions of Articles 1 and 2 "Convention on biodiversity of the Caspian Sea", the natural resources should be managed, so that future generations can also benefit from them. In connection with this, all Caspian littoral states should sparingly and carefully use biological resources of the Caspian. They should, using educational and research institutes, scrutinize the present state of the biodiversity of the Caspian Sea and widely disseminate obtained knowledge. Scientists from the Caspian littoral states should develop a common methodology for conducting an inventory of Caspian species and their habitats, to create new or to update old "red books" covering waters of the Caspian Sea and cis-Caspian region.
The governments of Caspian littoral countries should pay special attention to prioritization under exploitation of natural resources of the Caspian Sea. These riches can be conditionally divided into mineral and biological. Oil and gas fields, located at the bottom of the Caspian and in coastal areas, and saltern in the gulf Kara-Bogaz-Gol refers to the first. Fish, caviar and other sea products refer to the second. The leadership and people of the Caspian littoral states should make a correct choice. We believe that the priority should be given to biological resources, because the oil and gas riches of the Caspian will deplete sooner or later, but the biological riches are capable of reproduction and hence are practically inexhaustible and can yield a real and long-term prosperity to Caspian littoral states and nations. Preference of exploitation of biological resources would mean preference of real sustainable development. We do not deny the need to use oil and gas resources of the Caspian, however, we call to do it extremely carefully and sparingly in order to avoid damaging the unique biodiversity of this sea. In our opinion, this should our main objective.
The prevention of modification and deterioration of habitats and "hotspots" is the most effective way of preservation of biodiversity (Heywood and Watson, 1995; Norse, 1993). The concept of "hotspots" has gained currency under selection of protected areas (Norse, 1993). This term is also applied in other cases, for example, in regards to heavily polluted areas, but in our case it is used to identify an area with high biological diversity. This is a common objective at national and regional levels in the Caspian region.
There are three main aspects of the practical approach to preservation of biological diversity including:
The principles of preservations of biological diversity and sustainability are described in Articles 1 and 2 of the Convention on Biodiversity. Article 1 sets frameworks for the subsequent articles and also enacts, that:
" … Preservation of biological diversity, sustainable use of its components and honest and fair distribution of profit from use of genetic resources, including corresponding access to genetic resources and appropriate transition to a suitable technology, considering all rights on these resources and technology, and also appropriate funding. "
Article 2 determines sustainabile use as "use of components of biological diversity in such a way and to such an extent, which will not lead to a long-term drop of biodiversity, thereby supporting its potential, meeting needs and aspiration of the present and future generations."
To put it briefly, these articles tell about the need of availability of these resources for use by everybody including future generations. However, it can be achieved only under reasonable use of resources, i.e. under sustainable use, which can be determined only by means of education and researches. Therefore sustainability is linked to understanding and protection of biological diversity.
Many organisms are of low commercial value, but they are an integral part of well-balanced ecosystems and can be endangered by destruction of their habitats. This, in turn, can lead to loss of an unknown resource or even decline and then extinction of commercial species. For example, excessive exploitation and rapacious use of natural resources supporting these species such as oceans and forests. Sustainable use should also include restocking of species so that to increase population of overexploited species up to an "appropriate" self-sustaining level. Therefore each country should assess its biological diversity and to teach the public how to sustainably manage natural resources.
Correction of consequences of decades of destructive use of the Caspian Sea, which is a challenge, requiring tremendous efforts within next decades, will be followed by long-term activities on the basis of sustainability.
The Action plan of the United Kingdom (Anon, 1994) sets a number of goals, principles and tasks, listed below, which are the final part of Article 11 of the Convention on Biodiversity. They are valuable indicators of how to achieve sustainable use of natural resources and thus to shore up biological diversity. The indicators of this strategy are applicable to any region with rich natural resources.
Review of the purposes, principles and objectives of the Action Plan of the United Kingdom:
To save and to increase biological diversity and to assist preservation of global biodiversity by all possible means.
In the context of the Caspian Sea, the above objectives can be achieved by means of the actions presented in the text of this document - strategy and marked with the letter «A» (also listed in Appendix I).
To increase publicawareness on the importance of biodiversity and sustainability, the above principles and objectives all:
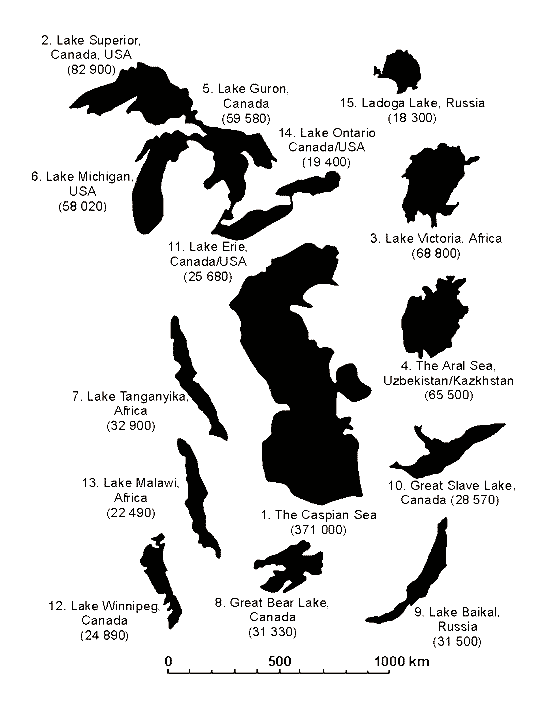
Fig. 1. Caspian Sea regarding the largest lakes of the World. In the brackets there is area in sq. km, area of the Aral Sea is given for 1960. (By Mary J. Burgis and Pat Morris “The Natural History of Lakes”, 1987)
The Caspian Sea is the largest lake on our planet. It is bigger than the Great American lakes and lake Victoria in Africa (Fig. 1) by the area of the surface. However, it is unique not only because of its size. As distinct from other lakes, the water of the Caspian is not fresh, but brackish. Each liter of Caspian water contains 10-13 grams of salt making this water unsuitable for drinking or irrigation. However, the comparison of the of the Caspian water to oceanic one shows that it contains three times less salt, than that of the World Ocean.
Why is the Caspian salty, and not fresh? The point is that the Caspian Sea is a remnant of the ancient the Thetis Ocean, or being more precise, its gulf - Parathetis. Some 50-60m years ago, Thetis Ocean connected the Atlantic and the Pacific Oceans. Gradually, due to movement of continental platforms, its connection, initially, with the Pacific Ocean, and later with the Atlantic, turning into an isolated water body. Thus, the salinity of the Caspian can be accounted for its genesis.
So why nowadays is the Caspian three times less salty, than the World Ocean? Under the isolation, the salinity of the Parathetis used to fluctuate. In hot and dry climatic phases with little precipitation, the Parathetis used to dry and to be divided into separate water bodies with more saline water than in the World Ocean. During cool and humid climatic phases with plenty of rainfalls, water bodies of the Parathetis used to be overflowed and again united becoming less saline. Glacier thawing exerted great influence on fall of water salinity in the Parathetis. A huge amount of thawed, fresh water lowered salt concentration, and for this reason, at present, the Caspian is three times less saline than the World Ocean.
The complicated history of formation of the Caspian Sea influenced its inhabitants. This giant lake can be compared to Australia. In our opinion, this comparison is not an exaggeration and is completely justified. Australia, as well as the Caspian, very early became isolated, and this isolation has ensured survival of many rare animals. Australia is glorified all over the world for its unique marsupial animals, which have managed to live through only because the separation of this continent from the rest of the world, and the evolution there has proceeded with its laws and even slowed down a bit. Therefore, many zoologists compare Australia with a lost world inhabited by living fossils. The same comparison is relevant for the Caspian, which has become famous all over the world for its unique sturgeon fishes. These Caspian fishes, as well as Australian marsupial, also are living fossils. The family of sturgeons already exists 200m years ago in the time of dinosaurs (Cousteau, 2000), inhabiting many ancient seas. However, later, in the course of the evolution, because of competition with bony fishes or for other reasons, sturgeons started dying out and survived mostly in the Caspian. Nowadays, more than 90 % of the world stocks of sturgeons live in this lake, which managed to survive only due its particular conditions. It is well known, that except for unique marsupial animals, there are many not less unique inhabitants in Australia, such as duck-billed platynus and echidna. And in the Caspian, except for sturgeons there are many other rare animals, such as, crustaceans and molluscs, which by their antiquity, of course, cannot be compared to dinosaurs, but are quite comparable to mammoths.
Considering the unique biodiversity of the Caspian and its similarity with Australia there are no doubts, that this unique lake and its inhabitants should be very carefully dealt with. Besides, the broad public should gain more access to results of Caspian studies and protected measures. Unfortunately, thus far attention has been paid in scientific, and especially in popular scientific literature to the Caspian, as distinct from Australia. The authors of this report will try to fill this gap.
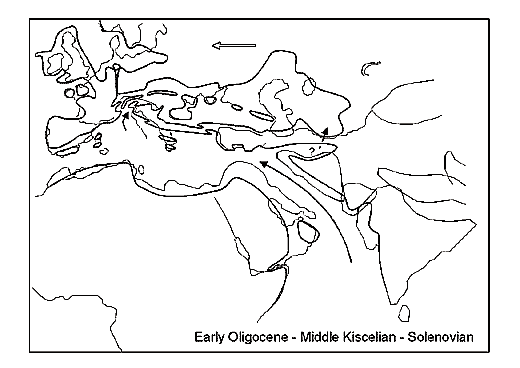
Fig. 2. (by Rögl, 1998).
Fig. 3. (by Rögl, 1998).
Aggregate basins, located from the valley of the river Rhone in Western Europe up to Central Asia in the Miocene are usually called the Parathetis in Paleontological literature. Traditionally three parts are distinguished: western, Central and East Parathetis. The first relates to the region of the Rhone, the second – the Pannonian or Middle Danube lowland, and the third – the Black and the Caspian Seas (Fig. 2, 3). In terms of modern mobilistic conceptions, this large continental water border formed due to movements of small continental platforms such as Iranian, Anatolian and Rhodopian. According to these notions, the basins of the Southern Caspian and the Black seas are considered as basins with oceanic crust, which enclosed as a result of movements of the above and some smaller other platforms.
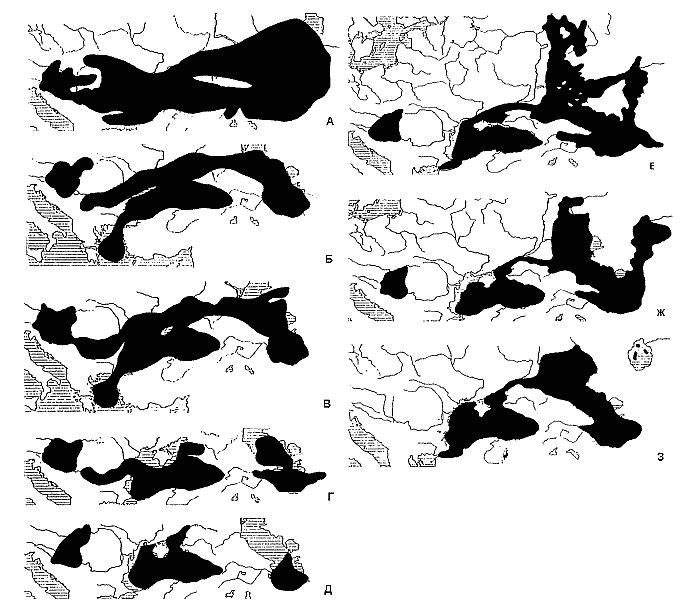
Fig 4. Palaeohydrography of south-eastern Europe ad south-western Central Asia in the late Miocene-Pleistocene (by Starobogatov, 1994 with changes and additions).
The Lower Sarmatian Sea existed in the region of Central and Eastern Parathetis some 15m years ago in the end of the Miocene. It used to occupy a huge area from the Pannonian lowland up to the Aral sea, and possibly extended further into Central Asia (Fig. 4). Although, the Lower Sarmatian Sea had the salinity of about 20 gr/l, i.e. much lower than that of the World Ocean and comparable to the present Black Sea salinity, the sea was inhabited by normal representatives of marine fauna.
Later, 11.5m years ago Pannonian brackish lake isolated. Endemic fauna, which lives under salinity of 12-15 gr/l, quite quickly formed in this lake.
The Upper Sarmatian Sea with lower salinity of about 6-17 gr/l emerged in another 1,5 million years, approximately 10m years ago. Own endemic brackish fauna, with species composition different from Pannonian Lake also formed here.
Some 8.5m years ago the Upper Sarmatian Sea transformed into the Miotic Sea with little bit lower salinity ranging from 6 to 15 gr/l. Because of lower salinity, the fauna of this sea became more distinct from the fauna of the Upper Sarmatian Sea. However, it is necessary to note that a kind of fauna consisting entirely of endemic species hadn’t formed yet (Neveskaya et al, 1986).
Later, approximately 7m years ago in the end of the Miocene, the Miotic Sea was substituted by Pontic Lake. Extremely rich endemic fauna immediately emerged in this lake. Most probably it hadn’t formed there independently, and was introduced from The Pannonian and Aegean basins (Eberzin, 1949), or only from Pannonian one (Taktakeshvily, 1977). The salinity of Pontic lake was close to that of the Meotic sea, however its fluctuations was less – ranging within 12-15 gr/l. It is necessary to note that only a few representatives of the Meotic fauna survived here. Probably it was not linked to changes of salinity since it hadn’t almost altered. Most probably the competition with invaders from the Pannonian and Aegean basins had played the major role.
Approximately 6m years ago or a bit later, Pontic Lake divided into Upper Pontic and Babajan lakes. The first used to occupy the Black Sea or Euxine basin, and the second was in the Southern-Caspian depression. The salinity of Upper Pontic Lake remained at the level of 10-15 gr/l, however in Babajan Lake, due to higher climate aridity, it increased up to 15-30 gr/l.
Some 5m years ago at the border of the Miocene and Pliocene, Pannonian lake, with emergence of intensive outlet into the Black Sea, and possibly in Aegean basins, completely freshened from 42 gr/l to fresh water and was settled with rich endemic freshwater fauna. Approximately 1m years ago Pannonian Lake completely drained and only a small lake Balaton, in which all representatives of endemic freshwater fauna completely perished remains on its place.
In the same time, also some 5m years ago, the Upper Pontic Sea transformed into Cimmerian Lake, which had lower salinity of 5-12 gr/l. Later, some 3.5m years ago, Cimmerian Lake was supplanted by Kuyalnits or Egriss Lake with almost same salinity. Approximately 2m years ago, it was replaced with Guriy Lake with lower salinity of about 5-8 gr/l. Thus, in this case, same as with the Pannonian basin, it was desalting but more smoothly. Because of this, one endemic brackish fauna was slowly supplanted by another, generically connected with it.
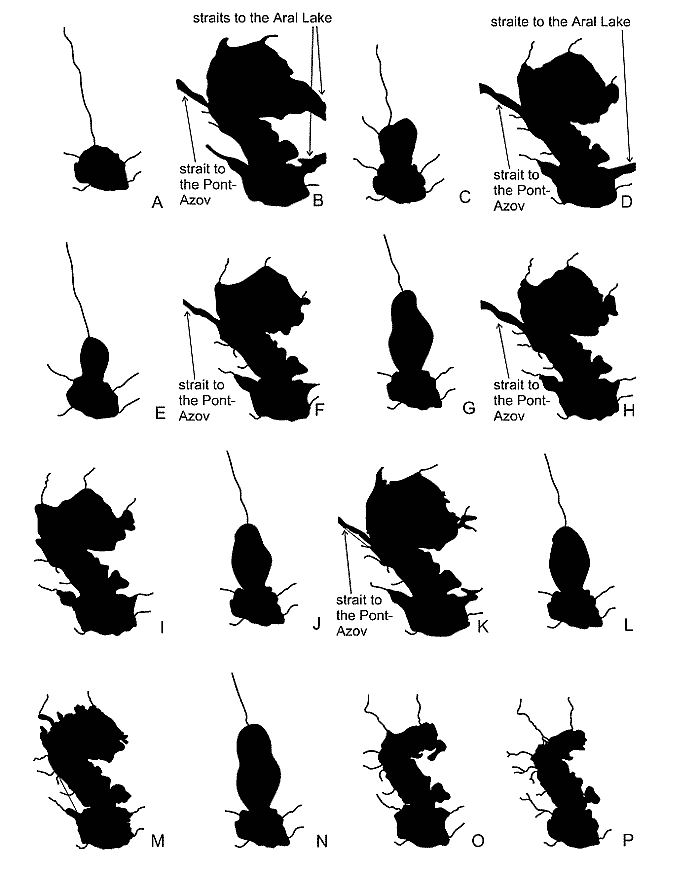
Fig. 5. Water bodies of the Palaecaspian (by Aladin, Plotnikov, 2000). A – Balakhanian; B – Akchagylian; C – Postakchagylian; D – Apsheronian; E – Turkianian; F – Bakuvian; G – Venedian or Ushtalian; H – the Early Khazarian; I – the Late Khazarian; J – Atelian; K – the Early Khvalynian; L – Enotaevian; M – the Late Khvalynian; N – Mangyshlakian; O – the New Caspian; P – the present.
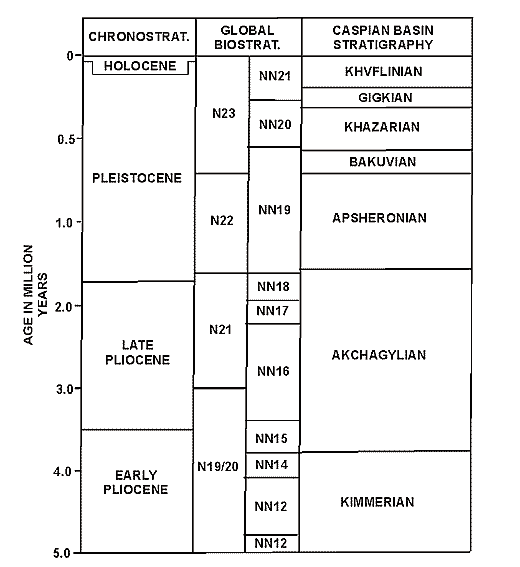
Fig. 6. Stratigraphy of the Caspian Sea Region (by Boomer et al., 2000).
Some 5m years ago, or a little bit earlier a hyperhaline lake, Balakhany or so-called productive strata reservoir formed in the Southern Caspian basin (Fig 5, 6). Such high salinization, up to 300 gr/l obviously occurred because of heavy water evaporations under conditions of extremely arid climate. Available data on the fauna of the Balakhany reservoir also convincingly testify hyperhalinity (Starobogatov, 1970, 1994).
About 3m years ago the climate dampened and freshwater runoff into Balakhany Lake soared. This led to formation of a huge brackish lake, Akchagyl with the salinity ranging from 5 gr/l to 12 gr/l, with rich endemic fauna of unknown origins (Starobogatov. 1970, 1994).
Akchagyl Lake in the Caspian basin was successively replaced by several water bodies with more or less consistent fauna. Five of these lakes were most important. Absheron lake with the same salinity as Akchagyl, 5-12 gr/l formed less than 2m years ago. Some 1.7m years ago, Absheron Lake was replaced by Baku Lake, which had the same salinity. Then 400 thousand years ago, Khazar Lake with the same salinity of 5-12 gr/l replaced it. After this, Khazar lake was substituted by Khvalyn lake, which had lower salinity ranging from 3 to 8 gr/l, approximately 100 thousand years ago. And finally, New Caspian Lake, which is practically the same reservoir, which we nowadays call the Caspian sea, formed in the beginning of the Holocene, some 5-7 thousand years ago.
Chaudine lake appeared in the Black Sea or Euxine basin approximately 900 thousand years ago, and later about 400 thousand years ago, it was supplanted by ancient Euxine lake. The salinity of these lakes was 5-8 gr/l, i.e. remained same with the former Guriy lake.
About 120 thousand years ago ancient Euxine Lake transformed into the Ashey Sea with higher salinity ranging from 5 to 12gr/l. Later, approximately 80-90 thousand years ago, it was replaced by the Karangat Sea with higher salinity of 15-20 gr/l. Restoration of the connection with the World Ocean resulted in an increase of the salinity. Because of this, marine species started to invade the Black Sea basin, driving endemic brackish Ponto-Caspian fauna closer to regions of estuaries and deltas of rivers with fresher water. However, some 50m years ago, this connection with the World Ocean was lost again, and the Karangat Sea was replaced with heavily desalinated New Euxine Lake, where the survived representatives of Ponto-Caspian fauna flourished. And, at last, on the edge between the Pleistocene and the Holocene, about 11-10 thousand years ago, the connection with the World Ocean was restored again, and the water body, which we nowadays call the Black Sea, appeared.
It is especially necessary to mark that only a unilateral exchange of faunas occurred when the Caspian and Euxine basins were connected The Caspian always shared its hydrobionts with the Pont, but not vise versa (Starobogatov, 1994).
The above scheme of development of the Caspian and of previous water bodies for the last 15m years was proposed by Y. I. Starobogatov (1994) based on the study of the evolution of bivalves, particularly dreissenids. Foreign authors (Degens, Paluska, 1979; Jones, Simmons, 1996) proposed similar development schemes. Although this scheme has a number of disputable moments, especially in terms of proposed magnitudes of paleohalinity, nevertheless, in comparison with other development scenarios, it seems to be the most substantiated and probable.
Reposing on this scheme, we will in-details consider the evolution of the Caspian, and bring forward magnitudes of paleohalinity, obtained in the course of study of microsculpture of conchostracs, investigated using a modified method of Rosenfeld-Vesper (Aladin, 1989; Aladin, Carpenters, 2000).
The Babajan reservoir arose some 6m years as a result of the under division of Pontic Lake into two parts. It used to occupy the Southern Caspian depression and was completely isolated from Upper Pontic Lake situated in the Black Sea or Euxine depression. Thus, the history of the Caspian as an isolated water body can be traced from the end of the Pontic epoch. Babajan Lake existed forapproximately 1m years and left a complex of Lower Pliocene deposits. If we use the dependence obtained for Cyprideis torosa (Àëàäèí, 1989) for calculation of magnitudes of paleohalinity, then the microsculpture of shells indicates the salinity of about 40-45 gr/l, which is by 15-25 gr/l higher than the salinity determined on the basis of specific composition of vegetative and animal fossils.
The Balakhany reservoir emerged about 5m years ago. It also used to occupy the Southern Caspian depression, but was obviously smaller than Babajan Lake. Balakhany Lake existed not less than 2m years and left a complex of Middle Pliocene deposits. For a special Balakhany layer with the thickness up to 4000 ì consisting of faunally diverse, sandy-clay and sometimes rudaceous deposits is distinguished on the Absheron peninsula. The lower part of the Balakhany layer corresponds to the Cimmerian layer of the Black Sea basin, and the middle and upper layers – to Kuyalnits. It is considered that the transformation of Babajan Lake into Balakhany Lake happened on the background of increasing climate aridity in the Middle Pliocene. There is no common opinion in regards to the salinity of Balakhany Lake. Initially, it was assumed that this lake used to be fresh water. However, freshwater mollusk fossils were found only at edges of the lake. This allowed P. V. Federov (1957, 1983) and Y. I. Starobogatov (1970, 1994) to assume that this lake was hyperhaline. In their opinion, which is substantiated with a careful analysis of malacofauna fossils in the Balakhany layer, the salinity of the lake could reach 300 gr/l. The character of microsculpture of Cyprideis torosa fossil valves testifies that the salinity of this ancient lake used to be at the level of 100 gr/l or even a little bit higher.
The Akchagyl reservoir emerged approximately 3m years ago, right at the beginning of the Pliocene and can be regarded as the brightest page in the history of the Caspian. The waters of Akchagyl Lake penetrated into the Aral depression, which at that time used to occupy the Black Sea or Euxine basin via the Manych hollow drained into brackish Kuyalnits basin. Extensive lowland stretches of trans-Caspian, Azerbaijan, Dagestan and Volga were flooded. It is believed that northern border of the reservoir lied in region of the river Kama. Obviously, that Akchagyl Lake had the highest level and area, and by its sizes considerably exceeded, for example, Khavalyn Lake, which emerged in the Later Pleistocene epoch. Akchagyl Lake existed for a bit less than 1m years and left a thick complex of deposits relating to a lower layer of the Upper Pliocene. Akchagyl strata were discovered in the beginning of the 20th century by N. I. Andrusov, who showed that they contain fossil fauna of a semi-marine type. This fauna originally lived under the salinity of 20-25 gr/l, however, later, as a result of large inflow of fresh waters, Akchagyl lake desalinized up to 5-12 gr/l. The character of microsculpture of shells of Cyprideis torosa testifies about very low paleohalinity, not higher than 5-6 gr/l. Thus, the paleohalinity of Akchagyl Lake, reconstructed using this method, appears to be a little bit lower than that determined using fauna fossils, mainly, molluscs.
Speaking about Akchagyl fauna and flora, it is necessary to note that there are two points of view on their origins. In the first case, when a semi-marine type is postulated, implying a genetic connection with Sarmatian fauna and flora is implied. In the second case, a marine type is defended assuming introduction of fauna and flora from the Arctic Ocean, Persian Gulf, Indian Ocean or Mediterranean Sea and Atlantic Ocean. The obtained data on rather low - 4-6 gr/l salinity in Akchagyl Lake prove that Akchagyl fauna and flora is mostly of semi-marine origins rather than marine. This point of view is also supported by the fact that endemic development of Caspian malacofauna in the Pleistocene was always characterized by survival of more ancient relicts in successive basins (Starobogatov 1970). In our opinion, basic elements of Akchagyl fauna and flora lived in Balakhany Lake, which later freshened and turned into a huge Akchagyl reservoir.
The post-Akchagyl reservoir appeared more than 2m years ago. It used to occupy only basins of the Middle and Southern Caspian, and it should be regarded as the maximum regression of the Akchagyl basin. It is difficult to say how long post-Akchagyl lake existed, as its deposits have been very poorly investigated. Most probably, the low level occurred for a short period, approximately 50-150 thousand years, and it was supplanted by the next transgression. Unfortunately, we did not manage to find valves of Cyprideis torosa in available collections, in order to determine paleohalinity of this lake based on their microsculpture. However, it is possible to suggest with confidence that the salinity of post-Akchagyl lake was much higher than that of Akchagyl, but lower than the salinity of Balakhany.
The Absheron reservoir emerged approximately 2m years ago. By its sizes, the lake was less than Akchagyl, however, its waters also penetrated into the Aral basin and drained into the Black Sea or Euxine depression which was accupied by the Guriy basin at that time. The cis-Caspian lowland was completely flooded and the Kura lowland and Karakum were partially inundated. Absheron Lake existed for more than 1m years and left a thick complex of deposits relating to an upper layer of the Upper Pliocene. Fauna fossils show that it had similar salinity to Akchagyl lake i.e. within 5-12 gr/l. It is also believed that the waters of Absheron Lake occupying the Aral basin were the most desalinized the salinity of about 5 gr/l. Those representatives of Absheron fauna, which were able to survive the greatest level ofdesalinization, occured here (Federov, 1983; Rubanov et.al. 1987). The character of microsculpture of valves of Cyprideis torosa shows the salinity level of about 7 gr/l. The materials from Absheron deposits, discovered in northern regions of the Aral basin, were analysed in addition to shells from the Caspian basins. These shells were found in detrital limestone of the Absheron epoch in the region of the gulf Shevchenko and peninsula Kokturnak of the Minor Aral Sea. The microsculpture of valves of Cyprideis torosa corresponds to the paleohalinity of 3-4 gr/l, and even about 1 gr/l.
The Turkan reservoir emerged a bit less than 2m years ago. It developed on the background of climate aridity and drastic reduction of freshwater inflow. Obviously, by its sizes, it resembles the post-Akchagyl reservoir i.e. occupied only basins of the Middle and Southern Caspian. Abrasive surface of Turkan lake is situated at the depth of 200-300 ì of the present Caspian Sea (Aladin, Carpenters, 2000). However, it would be a mistake to consider that the level of this ancient water body was so low. In Federov’s opinion (1983), such deep location is accounted for recent tectonic subsidence, and the real difference of levels of the present Caspian and Turkan lake does not exceed 100-150 m. Apparently, this lake, as well as post-Akchagyl, existed for a rather short period of time. Such a low level could have persisted for some scores and may be hundreds millennia, and it was ensued by the next transgression. Speaking about Turkan Lake, we should especially note that original Caspian brackish fauna of molluscs appeared in this lake for the first time. Thus, the turning point in development of fauna took place during a regression, instead of a transgression (Federov, 1983). The microsculpture of valves of Cyprideis torosa refers to the paleohalinity of 26-30 gr/l.
The Baku reservoir emerged later, some 1.7m years ago, on the background of climate moistening and cooling. It was less than Absheron Lake, and its waters did not penetrate into the Aral basin, but along the Manych hollow drained into the Black Sea or Euxine basin, where at this time the Chaudine basin was situated. Baku Lake occupied western stretches of lowland Karakum, completely Caspian and partially Kura lowland. It existed for about one million or half-million years, leaving a thick stratum of marine deposits, which was named the Baku layer. This layer is of the Quaternary age, and these deposits are usually compared to those of Likhvin glaciation on the Russian plain. However, we should note that lower part of Baku layer, probably, belongs also to an earlier period. Baku Lake went through three transgressions and two regressions. The long developing Early Baku transgression followed by the Late Baku and Urunjick transgressive phases. These phases were divided by two small regressions: post Early Baku and post Late Baku. It is considered that Baku lake had the biggest surface during the Urunjick transgressive phase and Early Baku had the smallest surface. The Late Baku transgressive phase took an intermediate position. As it was mentioned above, during all three transgressive phases, Baku Lake was bigger than the present Caspian and use to discharge its waters into the Chaudine basin. However, this discharge ceased during the Post Early Baku and Post Late Baku regressive phases. We can assume that during a low level period, the surface of Baku Lake was to that of the present Caspian Sea or even a little bit less. Unfortunately, because of short duration of these regressive phases of Baku Lake, it is very difficult to identify precise boundaries of the reservoir. Speaking about Baku Lake, it is necessary to note that except for the rivers, traditionally feeding the Caspian, such as the Volga, Ural, Emba, Atrek, Kura, Samur, Terek, Kuma etc., the Amu Darya also used to be its tributatry. At that time, this river did not flow into the Aral, but flowed through the lowland Karakum and entered Baku Lake at eastern part of the cis-Balkhan lowland. The microsculpture of valves of Cyprideis torosa from transgressive sediments of the Early Baku deposits refers to the paleohalinity of 8 gr/l; from Late Baku - 7.5 gr/l; from Urunjik - 6.5 gr/l. The microsculpture of valves from regressive sediments of post-Early Baku deposits refers to the paleohalinity of 10-15 gr/l, and from post-Late Baku - 8-12 gr/l. Reconstructed paleohalinity of Baku Lake appears to be a little bit lower than that determined from fauna fossils and, first of all, from mollusc fauna (Fedorov, 1957, 1980, 1983). It is considered that it was lower than 12 gr/l and hardly exceeded 13-14 gr/l. Our data show a wide swing of fluctuations of salinity ranging from 6.5 gr/l up to 15 gr/l, and the average salinity amounts to 8.3 gr/l.
The Vened or Ushtal reservoir arose approximately half-million years ago in the second half of the Early Pleistocene. Climate warming and aridisation occurred at that time, resulting in a decrease of river runoff and reduction of rainfalls. This was the reason for the decrease of water levels by 40-60m below the present level of the Caspian. The depression of the Northern Caspian completely dried up and waters of Vened or Ushtal lakes occupied the basins of the Middle and Southern Caspian. This lake existed for a rather short period of time. Apparently, such a low level didn’t maintained for more than 50 thousand years. The microsculpture of valves Cyprideis torosa refers to the paleohalinity of 18 gr/l.
The Khazar reservoir arose about 400 thousand years ago. It was a bit less than the Baku reservoir and, only at the very beginning, during the greatest transgression slightly exceeded the Upper Baku basin. The level of Khazar Lake experienced repeated fluctuations, with a generally trend shrinking. Originally, this lake had such a high elevation that its waters discharged into the Black Sea depression, where at this time located the Euxine-Uzunlar or Ancient Euxine basin. However, in the midst of the existence of Khazar Lake, its level even fell below the level of the present Caspian. This lake existed for approximately 300 thousand years and during this period it left a thick complex of brackish deposits, which is called the Khazar layer. Three groups of deposits are usually distinguished within this layer. The first group refers to the Lower Khazar transgressive phase, the second – to the post Lower Khazar regressive phase and the third – to the Upper Khazar transgressive phase, which gradually followed by a small regressive phase.
The Lower Khazar basin developed on the background of climate cooling and moistening, and as it has been already noted, its level was a little bit higher than that of the Upper Baku basin and excessive waters were discharged into the Euxine-Uzunlar basin. Thawing of the Oka glaciation of the Russian plain or Mindel occurring at that time coincided with this transgression. The Lowe Khazar basin, which was gradually shrinking, is usually subdivided into early, middle and late. The Early Lower Khazar basin experienced paleosingil transgression, the Middle - singil, and the Late - kosozh. Based on malacofauna fossils, in the end of paleosingil transgression, thawing of Mindel led to complete freshening of the whole basin (Fedorov, 1957). Obviously, the Early Lower Khazar basin was the most desalinized and the coldest, and its level exceeded the M.S.L. by 50 and more meters. According to data of paleobotanics, at that time, forests of humid and cold climates dominated in the coastal zone, average annual temperatures were lower by 6-100C (Aladin, Carpenters, 2000). Approximately 300 thousand years ago, when the Late Lower Khazar basin experienced the kosozh transgression caused by thawing the Dnieper (Riss) glaciation, freshening had already become less intensive and the water was a little bit warmer. If one judge on the basis of the range of development of trigonoid Didacna, then salinity then was about 11 gr/l (Fedorov, 1983).
The post Lower Khazar basin emerged on the background of certain climate warming and its level was even a little bit lower than the level of the present Caspian. It is quite difficult to state how long did this basin existed and how low was its level, because of its thin and low fossil sediments. Most probably, it existed for some scores of millennia and its level was below the present level by no more than several meters.
The Upper Khazar basin existed in the beginning of the Late Pleistocene under conditions of continuing climate warming, which eventually became hot with certain features of arid climate. Its level was lower than the level of the Lower Khazar basin, and there was no outlet to the Manych depression. However, during the maximal transgression, the Upper Khazar basin used to reach the level of 40-50 ì above the M.S.L., by that time closely approaching the point of overflow into the Black Sea or Euxine depression, where the Karangat basin was situated. The Upper Khazar basin, which was gradually lowering, is usually subdivided into the early, which emerged some 200 thousand years ago, and the late, which emerged some 100 thousand years ago. The salinity of the Upper Khazar basin, determined By development of the didacnas of the crassa group was a little bit higher than that of the present Caspian, not exceeding 14-15 gr/l (Fedorov, 1957). It is necessary to note that the Late Upper Khazar basin was the most saline among all Pleistocene transgressive basins – ancestors of the Caspian. Some authors (Starobogatov, 1994) also suggest that large and massive shells of Didacnas, found in great numbers in deposits of the Upper Khazar basin, testify of a higher water temperature in it, in comparison with the Lower Khazar basin.
The microsculpture of valves of shells of Cyprideis torosa from the Early Lower Khazar deposits refers to the paleohalinity of 5 gr/l; from the Middle Lower Khazar - 8 gr/l; from the Late Lower Khazar - 9 gr/l; from the post Lower Khazar - 15 gr/l; from the Early Upper Khazar - 10 gr/l; from the Late Upper Khazar - 13.5 gr/l. Thus, the reconstructed, by this method, paleohalinity of Khazar lake corresponds well with similar data obtained during the study of fossil molluscs.
The Atel reservoir emerged some 50 thousand years ago under conditions of arid climate. Its water used to cover only the depressions of the Middle and Southern Caspian. Apparently, the level of Atel Lake was lower than that of the present Caspian by 50m. Obviously, that this lake, as well as other regressive water bodies – ancestors of the Caspian existed for a short period. Probably, a level, lower than the present, with full dessication of the Northern Caspian remained for only a few millennia. We should note that the regression continued, under subsequent climate cooling and moistening. Arid conditions occurred only at the beginning of the life of Atel Lake. Its further history passed by under more cold and humid climatic conditions. In any case, cold-loving flora and mammoth fauna gained broad distribution in lower stretches of the Volga during that time. As Atel Lake existed for a rather short period of time, its sediments are rather thin. The microsculpture of valves of Cyprideis torosa refers to the salinity of Atel Lake of 20-22 gr/l.
Speaking about Atel Lake, it is necessary to tell that the most significant breaking point in the evolution of both water and land organisms during the whole Quaternary period occurred during its lifetime. Stratigraphic data convincingly confirm that the most essential changes in the fauna and flora related to one geological stage coincide with the Atel regression.
The Khvalyn reservoir emerged approximately 50 thousand years ago. It was the largest Pleistocene reservoir, however, inferior only to Akchagyl and, probably, Absheron lakes. The maximum level of Khvalyn lake reached the level of 45-48 ì, and its waters used to be discharged into the Azov-Black Sea depression by the Manych hollow, where the post Karangat basin was situated by that time. The discharges proceeded even under lower levels. Two main phases of this discharges occurred: under the levels of 45-48 ì and 22-25 m. There is no single opinion concerning the time when Khvalyn lake reached its maximum levels. It is believed that this took place some 45 thousand years ago (Fedorov, 1957), however, results of radiocarbon analysis of carbonaceous and vegetative materials indicate to a younger age of this event - 10.8-19.0 thousand years ago. The level of Khvalyn Lake experienced repeated fluctuations, and a considerable regression occurred in the middle of its life. It is called the Yenotayevskaya regression and during this period the surface of the lake was less than that of the Caspian. Khvalyn Lake existed for a rather short period, a bit more than 40 thousand than years, and during this spell it left a complex of brackish deposits, which are called the Khvalyn layer. This layer is considered to be corresponding to the lower half of the upper Quaternary system. The Khvalyn layer can be divided into three deposit groups. The first group refers to the Lower Khvalyn transgressive phase, the second with the Post Lower Khvalyn or Yenotayevskaya regressive phase and the third – the Upper Khvalyn transgressive phase, which was gradually followed by a short regressive phase.
The Lower Khvalyn basin formed during thawing the Early Valday Glaciation. As it was noted before, it was the largest Pleistocene reservoir with considerable general desalinization. The climate moistening led to an increased runoff in all rivers flowing into the Lower Khvalyn reservoir. The discharge of the ancient Amu Darya also increased. However, approximately during the spell of the maximum level of the Lower Khvalyn basin, the Amu Darya overflowed so much, that of its waters have turned toward the Aral depression, which was by that completely dry time. In a while, this depression became completely filled with water so that it started to drain into the adjacent Sarykamysh basin. Gradually, the waters of the Aral and Sarykamysh united, and after that an outlet into Khvalyn Lake emerged. The channel of this outlet is called Uzboy. Thus, the waters of the Amu Darya, which passed in transit through the Aral and Sarykamysh, eventually, reached the Caspian basin again. Unfortunately, now it is very difficult to determine how long it took to fill the Aral and Sarykamysh, before the discharge trough Uzboy emerged. Different authors differently evaluate the duration of filling of the Aral-Sarykamysh Lake and origination of the Uzboy outlet. Certain authors believe (Kes, 1960), it arose rather quickly, i.e. soon after the peak of the Lower Khvalyn transgression. Other researchers (Rubanov et. al. 1987; Kvasov, Mamedov, 1991; Aladin, Carpenters, 1995) reckon that this occurred in the end of the Upper Khvalyn transgression or even later.
Based on data obtained during the study of fossil molluscs, we can conclude that a considerable drop of temperatures and abundant inflow of fresh waters occurred during the period of the Lower Khvalyn transgression. All this caused loss and partial regeneration of Khazar mollusc fauna. As a result, new species appeared and the Khvalyn complex of mollusc fauna formed (Fedorov, 1957). It is necessary to stress that by that time, the mollusc fauna acquired almost a modern appearance. As for the reconstruction of mollusc fauna, it is considered that the paleohalinity during the peak of the Lower Khvalyn transgression ranged within 6-7 gr/l.
The post Lower Khvalyn or Yenotayevskiy regressive basin formed under rather dry and warm conditions, probably, during the interglacial epoch. The level of this basin lowered even below the present one. It is considered that certain warming of water and an increase of salinity had a favorable bearing on mollusc fauna. However, it is difficult to say how correct these suppositions are, because of the poor fossil content of these deposits. For the same reason, it is very difficult to judge about the lifetime of the Post Lower Khazar basin, and also about how low its level was.
The Upper Khvalyn basin emerged 20-11 thousand years ago under rather arid climate conditions, which were more humid and colder than the present one. Its level was lower than that of the Lower Khvalyn basin and it was always completely isolated from the New Euxine basin, which was occupying the Black Sea depression. It is considered that the maximum level of the Upper Khvalyn transgression was close to the initial level. In the opinion of some researchers (Fedorov, 1957), the peak of this transgression coincides with the end of the last glacial period, i.e. 9-10 thousand years ago. However, other authors (Mayev et.al. 1983) supported by the results the radiocarbon analysis of shell carbonate, suggest a smaller age - 6.8-8 thousand years ago. After the peak spell, the Upper Khvalyn reservoir gradually began to shrink. Development of molluscs shows the salinity of the Upper Khvalyn basin was a bit lower than the present one and did not exceed 10 gr/l. As for the mollusc fauna of that period, it was much poorer than that of Lower Khvalyn and almost did not differ from the present one.
The microsculpture of valves of Cyprideis torosa from the Lower Khvalyn sediments refers to the paleohalinity of 4 gr/l, from the post Lower Khvalyn - 12.5 gr/l, and from the Upper Khvalyn - 8 gr/l. Thus, the paleohalinity of Khvalyn lake determined, by this method, appears to be a little bit lower than that determined by fossil fauna, and, first of all, by mollusc fauna (Fedorov, 1957, 1980, 1983).
The Mangyshlak reservoir formed about 7.5 thousand years ago in the beginning of the Holocene. It emerged in the period of postglacial warming and climate aridity. Sometimes its formation is associated to an earlier period of time and dated to the late Pleistocene (Fedorov, 1957). Its waters used to cover only depressions of the Middle and Southern Caspian. The levels of Mangyshlak Lake was 20-23 ì lower than those of the present one. This lake, as well as other regressive reservoirs – ancestors of the Caspian, existed for a short period of time. Most probably, this level, when the Northern Caspian entirely desiccated, did not lost for more than 2 thousand years. Since Mangyshlak Lake had a rather short life, its sediments are not thick. Fortunately, it was possible to find in these thin sediments molluscs from the group Didacna crassa and D. Baeri that testifies about a rather high salinity of about 15-17 gr/l.
In regards to the Mangyshlak regressive reservoir, it is necessary to note that during the period of its existence Cardium edule (= Cerastoderma lamarcki) invaded this region. It happened some 7-5 thousand years ago, when there was no connection with the Azov-Black Sea basin and so natural settlement of the mollusc was absolutely impossible. There are two hypotheses of the invasion of Cardium edule (= Cerastoderma lamarcki). According to the first (Fedorovich, 1952), the molluscs were brought in by birds, and according to the second – by man (Fedorov, 1957). In our opinion, the second hypothesis looks more probable, since neolithic tribes migrated by the lacustrine-riverine system of the Manych hollow at this time, and these tribes used these molluscs as food. Later, with the tribes of ancient people Cardium edule (= Cerastoderma lamarcki) also invaded the Aral through the system Uzboy-Sarakamysh. The microsculpture of valves of Cyprideis torosa refers to the paleohalinity of 18-20 gr/l.
The New Caspian reservoir emerged approximately 5-6 thousand years ago. The level of this lake during its peak was lower than the M.S.L., but higher than the level of the present Caspian. Most probably, its altitude was lower than elevation of the World Ocean by 20-23 ì. Results of the radioisotope analysis of carbonaceous and vegetative materials indicate that the maximum age ranges from 6.4 + 0.55 thousand years ago up to 5.39 + 0.11 thousand years ago. However, majority of points refers to the interval of 2-3 thousand years ago. During its lifetime, this reservoir left sediments, which are called "New Caspian", or "Post Khvalyn" layer. This layer contains marine and continental deposit of the Holocene. The microsculpture of valves of Cyprideis torosa refers to the paleohalinity of 8-13 gr/l. The magnitude of paleohalinity determined with help of analysis of the microsculptures of valves of C. Torosa, as a rule, is a little bit lower than that obtained from the analysis malacofauna. Since the method of determination of paleohalinity using mollusc is a qualitative one, and the method of microsculpture analysis of shells of conchostracs - is a quantitative one, so in our opinion the data obtained using the latter method, apparently, is more trustworthy.
It is necessary to note that researchers from the universities of Moscow and Tel Aviv (Bratanova, Svitoch, 1997; Svitoch, Yanko, Bratanova, 1997) have recently conducted a careful research of paleohalinity of Pleistocene reservoirs – ancestors of the present Caspian, using the fauna of foraminifers and ostracods. These researchers carried out an in-depth analysis of marine Pleistocene deposits in Azerbaijan and lower stretches of the Volga, selecting marine, brackish and freshwater concentrations of conchostracs and rhizopods. The richest concentrations of remnants of these organisms were found in Baku, Urunjik and neo-Caspian sediments. As distinct from this, the shells of ostracods and foraminifers were found extremely rarely in the Guriy and Early Khvalyn deposits. In opinion of these authors, at these periods of life of the ancient Middle and Southern Caspian, the salinity of the sea used to change by several gr/l and it was close to the present salinity of the Caspian (about 13 gr/l). As for the ancient Northern Caspian, here because of the flow of the ancient Volga and Ural, salinity fluctuations were higher, and their swing used to reach 10 gr/l. These researchers distinguish two different tendencies for the transgressive Pleistocene epoch: salinization and desalinization. In their opinion, the trend of desalinization was revealed at early stages of transgression in Baku time (at western coast), and trend of salinization - at early stages of transgression in Khazar time (in the region of lower stretches of the Volga). These authors also emphasize that, in accordance with the microfauna of Urunjik and Holocene deposits, the rhythmic changes of salinity were observed during these periods of existence of the ancient Caspian.
The above mentioned data on the paleohalinity of Pleistocene reservoirs - ancestors of the present Caspian, obtained from analysis of the fauna of ostracods and foraminifers, in general, comply with our data obtained from the analysis of the microsculpture of shells of Cyprideis torosa. However, as well as in the case of the determination of paleohalinity on fauna of molluscs, the data obtained using the technique of Rosenfeld-Vesper seems to be more precise.
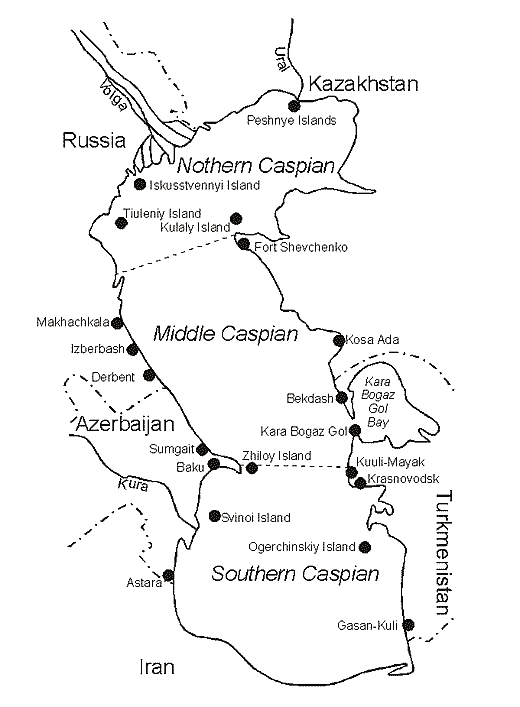
Fig. 7. Caspian Sea water area (by Rodionov, 1994).
The Caspian Sea - is a lake with no outlets, which is washing shores of the five countries: Azerbaijan, Iran, Turkmenistan, Kazakhstan and Russia (Fig. 7). The length of coastline makes 5580 kms, and if one takes into account the coastal line of Caspian islands, then it amounts to 7000 kms. Of these 600 kms lie in Azerbaijan, 725 kms - Iran, 1200 kms - Turkmenistan, 2300 kms - Kazakhstan, and 755 kms – Russia.
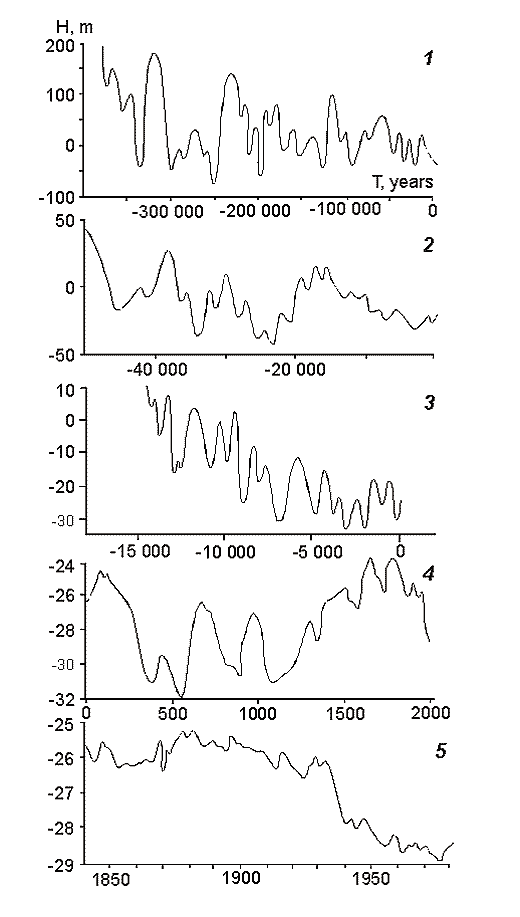
Fig. 8. Changes in the Caspian Sea level (by Maev et al., 1986).
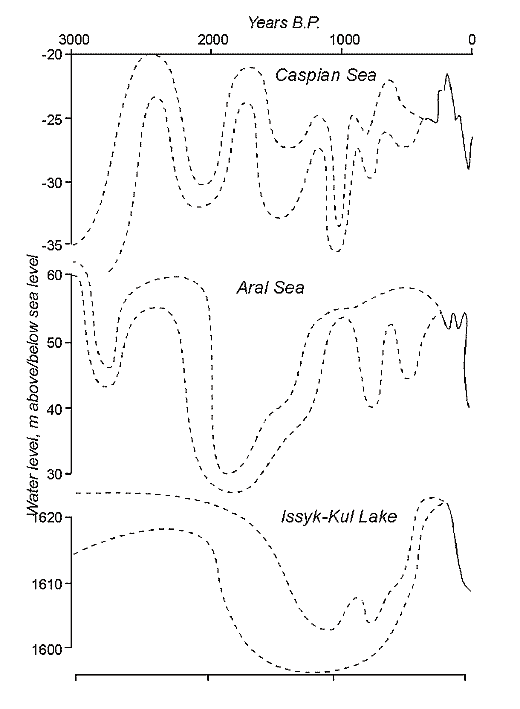
Fig. 9. Water level changes of the largest enclosed lakes in Central Asia during the last 3000 years by documentary and proxy data. Dotted bars represent a variety of estimates, whereas solid lines show more reliable data.
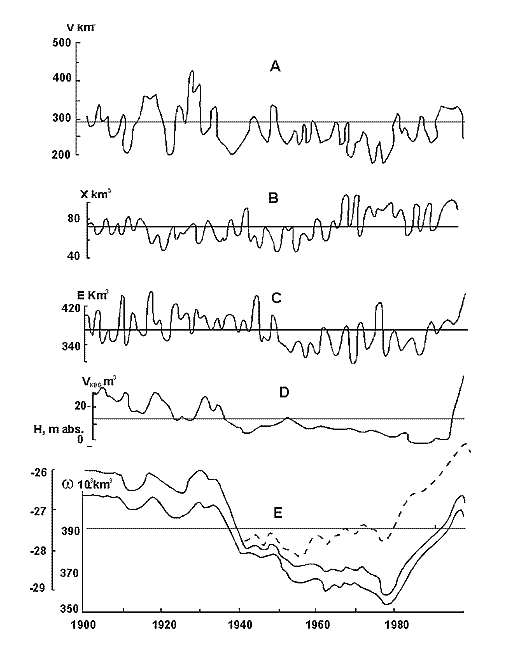
Fig. 10. Changes of the Caspian Sea water balance, level an area from 1900 till 1996. Dotted line – reconstructed level (without taking water).
This is the largest lake in the world by its surface area. The level of the Caspian is lower than the M.S.L., however, it is fluctuates depending on the water balance. If the balance is positive then the level of the Caspian is rising, if negative – lowering (Fig. 8-10). Because of inconstancy of Caspian levels, its area is also inconstant. Usually, the following geographical coordinates are provided for the Caspian Sea. It lies between 47013’ and 36034’ 35‘’ of northern latitude and between 46038’ 39’’ and 54044’ 19’’ of eastern longitude. The Caspian is meridiannally elongated, and its length from the north to the south is 1204 kms (Zenkevich, 1963). According to data published by I.S. Zonn (2000), this length is even more and makes 1225 kms. The greatest breadth of the Caspian from the east to the west is 566 kms, however, in region the Absheron peninsula its breadth is only 204 kms. The average breadth of the Caspian from the west to the east makes 330 kms. The surface of the Caspian is equal to 436 000 êì2, and its volume is about 77000 êì3. In the Guinness Book of Records (1998) the maximum volume of the Caspian is indicated equal to 89600 êì3. The maximum depth of the Caspian is 1025 ì, and the average - 184 m.
The area the Caspian Sea is divided into three, approximately equal, parts: Northern, Middle and Southern. Although all these three areas have approximately equal areas, however, their volumes they are extremely different. The part of the Caspian liying to the north of the line: island Chechen - cape Tub-Karagan is the most shallow, and, therefore, the smallest by its volume. This region is called the Northern Caspian, and its area makes about 29 % of the entire area of the sea, though its volume makes less than 1%. According to the data produced by I.S. Zonn (2000), the area of the Northern Caspian varies from 92750 up to 126596 êì2, and its average volume makes - 900 êì3. The average depth of the Northern Caspian is 6 meters, and maximal depths do not exceed 10m. About 20 % of the area of the Northern Caspian have the depths less than 1 m.
The Middle Caspian is limited in the south to the line: island Zhiloy - cape Kuuly. The area of this part of the Caspian makes about 36 %, and its volume - about 35 % of the sea. According to the data produced by I.S. Zonn (2000), the area of the Middle Caspian varies from 133560 up to 151626 êì2, and the average volume makes 26400 êì3. The average depth of the Middle Caspian is about175 ì, and the greatest - 790 m.
The Southern Caspian has the largest volume - some 64 % of the total volume, and its area amounts to 35 % of the total area of the sea. The Southern Caspian is the deepest part of the sea with the maximal depth reaching 1025 m. According to the data produced by I.S. Zonn (2000), the area of the Southern Caspian is from 144690 up to 151018 êì2, and the average volume - 48300 êì3. The average depth of the Southern Caspian is 300 m.
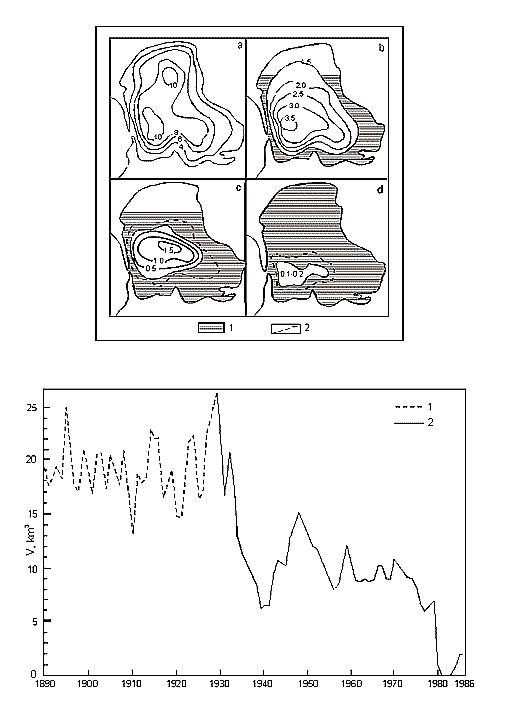
Fig. 11. A – Position of Brine Border and Depths (m) in Kara-Bogaz-Gol Bay (Terziev et al., 1986). Years: a – 1933, b – 1971, c – 1982, d – 1983 December. 1 – Dried bead of bay. 2 – Periodic inundation borders of salts zone. B – Long-term Inflow Changes of Caspian Waters intu Kara-Bogaz-Gol Bay (cu km/annum).
Except for the above three areas, the fourth is distinguished in the Caspian, which is a shallow gulf, Kara-Bogaz-Gol (Fig. 11) with maximal depths not exceeding 10m. Its area is about 15000 êì2, amounting to more than 3 % of the total area of the sea. However, the role of the gulf in the water balance of the Caspian is quite great. The point is that this shallow gulf is lower than the level of the sea approximately by 3-4 meters and due to this the sea constantly drains into it. Owing to this active drain, the gulf obtained its name. Translation from Turkic language of Kara-Bogaz-Gol means Black throat gulf. Kara-Bogaz-Gol as "the insatiable black throat", which constantly "drinks" the water of the Caspian, and this water, in turn, quickly evaporates on boundless extents of this shallow gulf. The gulf is connected with the Middle Caspian by a narrow strait, 110 up to 300ì across, and 8-10 kms long. Thus, this gulf is a large evaporator of the Caspian Sea playing an important role the water balance of the sea. In the end of the 19th and the first half of the 20th centuries, 20-25 êì3 yearly drained into Kara-Bogaz-Gol, and 10-15 êì3 in the middle of the 20th century because of siltation of the Caspian. Presently, 200-300 ì3 of Caspian water is discharged into Kara-Bogaz-Gol every second. Along with this water, some 130-150m tons of salt is brought in from the sea, which is 10 times the amount, which the Caspian receives.
As it was mentioned above, the level of the Caspian is not constant. The highest level registered during instrumental observations was recorded in 1896. By that time, the waters of the Caspian were at the level of approximately 25 ì below the M.S.L. It is possible to distinguish three periods in the 20th century: the period of relative stability, the period of water levels fall and the period of water levels rise. The period of relative stability was observed till 1917. However, starting from that year the level began to drop from -25.82ì, and by 1925 lowered up to 26.26m. During the following five years, the sea level rose again by 0.8 ì reaching 26.06m by 1930. A slow decrease started from that year and a drastic one from 1933. In the beginning of the Second World War (1941), the sea level was at the altitude of -27.88 m. During the war and the first years after it the level stabilized and fluctuated around the altitude of 27.96 m. A sea levels fall resumed in 1949 proceeding till 1977. The lowest levels in the 20th century were recorded that year -29.03 ì. However, an extremely fast rise began next year, and in ten years the water reached the elevation of -27.62 m. The Caspian stabilized again in 1995 at the level of -26.61 ì, and during subsequent years – the levels fell by several centimeters each year. Nowadays, the level of the Caspian is slowly decreasing and comes to the altitude of -27.20 m.
As a result of the sea levels decrease in the middle of the 20th century, the area of the sea has considerably shrunk leading to changes of its outlines. Mainly, it related to the Northern Caspian, as its shallow gulfs located in eastern part such as Mertviy Kultuk and Kaidak dried and turned into salinas in the beginning of the 40-s. In 1930 the area of the sea was 422000 êì2, and in 1970 - only 371000 êì2. Starting from 1978, the area of the sea increased again because of a sea levels rise, and the gulfs Mertviy Kultuk and Kaidak were filled in with water again.
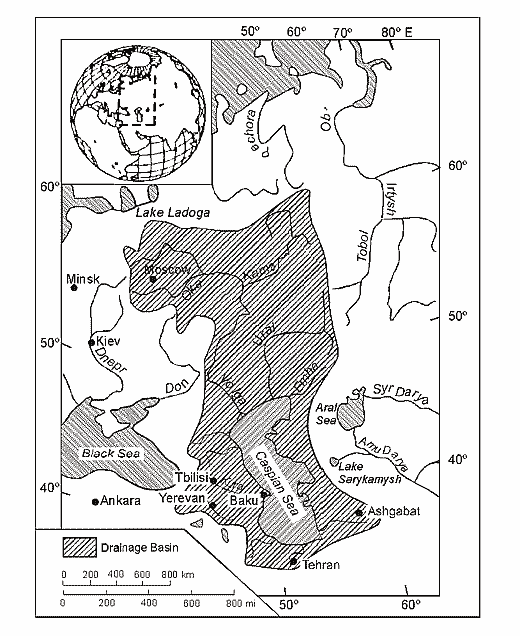
Fig. 12. The Caspian Sea basin (by Rodionov, 1994).
The area of the watershed of the Caspian Sea makes from 3.1 up to 3.5m kì2. It stretches for 2500 kms from the north on the south and for 1000 kms from the west to the east. The area of the Caspian itself makes only 12% of the total area of the watershed (Fig. 12). In accordance with Golubev’s calculations (Golubev, 1997), the catchment of the Caspian amounts to 10 % of all areas on our planet, which have no outlet. As it was mentioned above, five countries are located at the seashores of the Caspian. However, except for these five countries, other countries are located in the basin of the Caspian: Georgia, Armenia, Uzbekistan and Turkey. Thus, the total number of countries located in the basin of the Caspian is equal to nine. The water balance of the Caspian is mainly determined by river runoffs and rainfalls (it is its incoming part), evaporation and water outflow into Kara-Bogaz-Gol (it is its outgoing part). The ground water runoff into the Caspian is insignificant and this incoming component of the water balance is frequently disregarded. The most important part of the incoming part of the water balance is the river runoff of the Volga, which makes almost 80 % of the total riverine inflow. The incoming part is almost completely counterbalanced with evaporation, of which the discharge into Kara-Bogaz-Gol makes only 5%. It is interesting to note that the evaporation is 5 times higher than the rainfalls. In Zaikov’s opinion (1946), the temporal dynamics of the water balance, lowering and increase of its levels are mainly stipulated by the variability of river runoffs. More than 130 rivers flow into the Caspian, but only 9 of them have a delta. The average long-term runoff of the Volga passing through the Volgograd Hydroelectric Station is evaluated at the level of 251-254 êì3. The range of fluctuations during the whole period of instrumental observations has made more than 200 êì3 since 1881. So, for example, more than 380 êì3 of water passed by the Volga in 1926 and only 160 êì3 in 1921 and 1937. The delta of the Volga is the largest delta on the Caspian, it area is 19000 êì2. It starts in the place of the offshooting its branch Buzan from the main channel of the Volga, which is 46 kms north of Astrakhan. The average length of the delta of the Volga from the north to the south is 120 kms, and its breadth bordering the sea is about 200 kms. There are more than 500 branches in the delta of the Volga. Another large river flowing into the Caspian at the territory of Russia is the Terek (discharge 8.5-11.4 êì3). Its delta makes about 9000 êì2 and is almost half the delta of the Volga. The river Sulak (discharge 3.6-4.0 êì3) has a very small delta - only 70 êì2. This river also flows into the Caspian Sea at the territory of the Russian Federation. Two big rivers – the Kura (discharge 13.0-15.5 êì3, delta - 204 êì2) and the river Samur (discharge 2.7 êì3, delta 80 êì2) flow into the Caspian in Azerbaijan. Eastern coast of the Caspian is almost absolutely deprived of a hydrographic network. At present, only the river Ural (discharge 6.6-8.1 êì3) flows into the Caspian at the territory of Kazakhstan. Its delta is about 500 êì2, which is almost four times less than the delta of the Volga. Nowadays, another Kazakhstan river, the Emba, reaches the Caspian only during high water years, though in the beginning of the 20th century it had an extensive delta with several branches. The fall of the Caspian levels in the 1930s stopped the Emba’s inflow into the Caspian. In Turkmenistan, there is only one river, the Atrek flowing into the Caspian. This river often dries in its lower stretches, and its waters reach the Caspian only in spring. The average yearly runoff of the Atrekà is less than 240m m3. The rivers of Iran provide 5 % of the total river runoff into the Caspian. The largest river of Iran flowing into the Caspian is the river Sefidrud (discharge 3.93-4.67 êì3). This river has a delta of 1800 êì2, which is ten times less than the delta of the Volga.
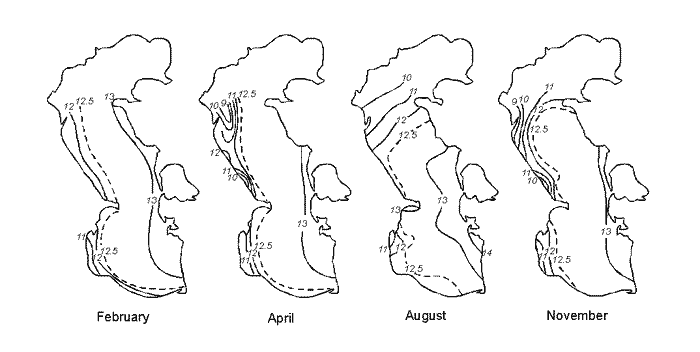
Fig. 13. Mean salinity (g/l) on the surface in the Caspian Sea: February, April, August, November.
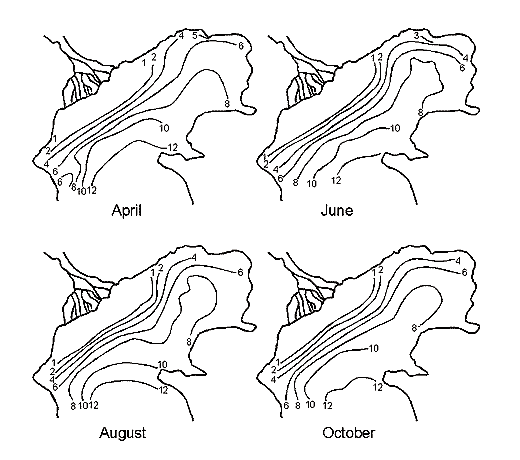
Fig. 14. Mean salinity (g/l) in the Northern Caspian Sea: April, June, August, October.
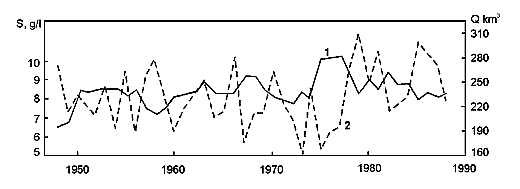
Fig. 15. Long-term changes in the salinity of the Northern Caspian Sea (1) and the annual Volga run-off (2).
The major abiotic parameter of the Caspian Sea is its salinity (Fig. 13-15). The average salinity of the Caspian is equal to 12.85 gr/l. However, all considered above areas of the Caspian: Northern, Middle, Southern and the gulf Kara-Bogaz-Gol differ with the salinity of their waters. The lowest concentration of salt is observed in the Northern Caspian. The average salinity of its waters is about 5-10 gr/l. However, in certain areas of the Northern Caspian adjacent to the deltas of the rivers Volga, Ural and Terek, the water salinity is much lower than the above values and fluctuates within 2-4 gr/l. In the avant-deltas of these rivers, the water of the Northern Caspian can be consider as fresh, since its salinity is less than 0.5 gr/l. In shoals of eastern coast of the Northern Caspian, the water salinity can be a little bit higher than the averages - 5-10 gr/l. The point is that strong evaporation is observed in calm weather in the shallow gulfs of eastern coast, and, as a result, fast salinization up to 15-20 gr/l occurs. Water salinity up to 30 gr/l and even more can be observed in shallow gulfs of the Northern Caspian such as Mertviy Kultuk and Kaidak.
The salinity of the Middle Caspian is 12.7 gr/l. This salinity is reduced only in the region of the delta of the Sulak, where a lower magnitude can be observed. As eastern coast of the Middle Caspian has no river runoff, the amount of rainfalls is very low, and the evaporation is high, so the water salinity in calm weather in surface coastal waters can reach 13.0-13.2 gr/l.
The salinity of the Southern Caspian is 13 gr/l. As well as in previous cases, this salinity is lower in areas, adjacent to the regions of the deltas of the rivers Kura and Sefidrud, and also in the mouth of the river Atrek, when this river reaches the waters of the Southern Caspian. Besides, higher salinity can be observed at eastern and southern coasts. The water salinity of 13.2-13.4 gr/l is observed on shoals of the Turkmen coast.
The highest concentration of salt is observed in the gulf Kara-Bogaz-Gol. This gulf, as figuratively expressed K. M. Bear, is "an evaporating pan" of the Caspian. The evaporation rate from the surface of Kara-Bogaz-Golà amounts to 1500 mm a year, and the rainfalls in this region do not exceed 70 mm per year. For this reason, Kara-Bogaz-Gol is a huge evaporator of the Caspian, and as a matter of fact, its water is brine. The salinity of Kara-Bogaz-Golà reaches 300-350 gr/l and even higher. It is the only marine saltpan producing mirabilite, halite and astrakhanite. Inflowing water brings some 130150m tons of salt i.e. 10 times more, than the Caspian itself receives. For this reason, the gulf Kara-Bogaz-Gol can be called a natural desalter of the Caspian Sea. Without this gulf, the present salinity of the Caspian would be much higher. A huge amount of mineral substances is accumulated at the bottom of Kara-Bogaz-Golà due to evaporation and natural sedimentation of salts.
Analyzing the areas of the Caspian by their salinity, they can be subdivided into three types: oligo-mesohaline area of the Northern Caspian, meso-polyhaline area of the Middle and Southern Caspian and hyperhaline area of the gulf Kara-Bogaz-Gol.
The average salinity of the Caspian Sea is lower than that of the ocean approximately by a factor of three. However, the salinity of the Caspian waters is not the only difference from waters of the World Ocean, but also the salt composition. The water of the Caspian Sea is rather poor with sodium and chlorine ions and is rich with ions of calcium and sulfates. The difference in the ratio of salts of Caspian and oceanic waters has arisen due to the isolation of the Caspian from the World Ocean. As a result, a gradual metamorphosis of Caspian waters under the influence of river runoffs took place.

Fig. 16. Salinity (g/l) on the longitudinal transect: a – February, b – August.
In open parts of the sea, salinity increases with the depth (Fig. 16). So, for example, if on the surface of the Southern Caspian, the water salinity is 12.7 gr/l, so at the bottom at the depth of 700 ì, the water salinity reaches 13.1 gr/l.
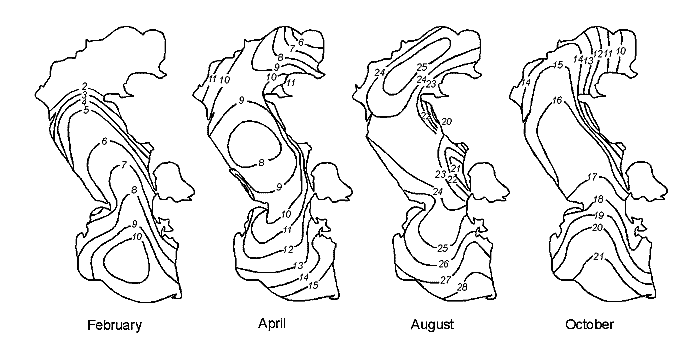
Fig. 17. Mean temperature (˚C) on the surface in the Caspian Sea: February, April, August, October.
The temperature regime of the Caspian Sea is rather unusual (Fig. 17). On the one hand, it is characterized by considerable temperature differences in wintertime between its northern and southern areas, and on the other hand, leveling of the temperature regime between the Northern and Southern Caspian in summertime. In general, the Caspian has a sharp continental climate. In wintertime, in the north of the Caspian the average monthly air temperature makes -8-100C. In the same time, in the south, air temperature remains positive about 100C. In the winter only the Northern Caspian freezes. In cold winters from January to March the border of an ice cover lies from island Chechen up to the peninsula Mangyshlak curving toward the north. The thickness of ice in the Northern Caspian can reach half a meter, and in the northeast even up to 70 cm. In this period, water temperature under ice can drop up to -0.50Ñ. In abnormally warm winters, the ice cover can be almost completely absent in the Northern Caspian. So, for example, during the winter of 2000, only a shore ice belt was found. The open areas the Northern Caspian were not cover with ice, and only individual ice fields floating in the sea were observed. In summertime, the air temperatures of northern and southern parts of the sea are leveled. The average air temperature in the Northern Caspian is 24-250C, only by 2-3 degrees higher than in the Southern Caspian.
Precipitation is distributed unevenly over the Caspian. In the average the Caspian receives some 100 mm of rainfalls per year. Coastal topography influences the balance of rainfalls over the Caspian. The minimal amount of rainfalls is observed at eastern coast, less than 90 mm, at northern - less than 300 mm, at western from 400 up to 600 mm, at southwestern - 1600 mm.
Water temperature in the Southern Caspian never drops below 13 degrees in wintertime, and in summertime it is usually increases up to 25 and even 300C.
Seasonal oscillations of water temperatures are more considerable in the Middle Caspian. In wintertime, the average temperature of surface waters is only 60C, and then it increases up to 250C by the midst of a summer.
In the Northern Caspian, seasonal changes of water temperature have a maximum character. As it was already noted, in wintertime the Northern Caspian is partially covered with ice, and water temperature is about 00C and even lower. In the middle of a summer, the average water temperature of the Northern Caspian is 240C. We should note that in calm weather the water temperature in shallow gulfs of the Northern Caspian could reach 350C. Similar and even higher summer water temperatures (up to 400C) are observed at eastern shoals of the gulf Kara-Bogaz-Gol.
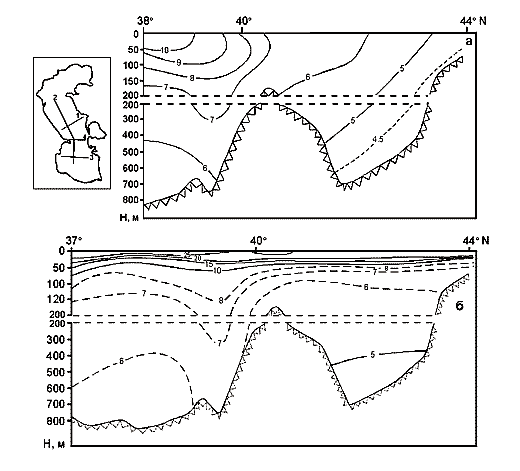
Fig. 18. Water temperature (˚C) on the longitudinal transect: a – February, b – August. In the insert are shown the hydrological transects: 1 – Divichi – Kenderli, 2 – Zhiloi – Kuuli, 3 – Kurinsky Kamen – Ogurchinsky, 4 – longitudinal.
Constant temperature is maintained at depths of the Caspian Sea both in winter and summer (Fig. 18). In the Southern Caspian at the depth below 150 ì the water temperature is 70C the whole year round, and at the depth below 500-600 ì - 60C. In the Middle Caspian at the depths below 150 ì, the temperature is about 6-50 C the whole year round, at the depth below than 400-500 ì it ranges between 4.5 and 50C.
In the Northern Caspian there is no water stratification by temperatures due to its shallowness. Only in calm weather it is possible to note difference in temperatures between the bottom and the surface. Similar homotermia is observed in the shallow gulf of Kara-Bogaz-Gol, though the difference of temperatures between the bottom and the surface and can be observed here in calm weather.
Thus, each of the four areas of the Caspian: Northern, Middle, Southern and the gulf Kara-Bogaz-Gol have its own certain features of the temperature regime.
There is a system of horizontal and vertical movements of water masses in the Caspian, as well as in any other water body. Surface currents in the Middle and Southern Caspian will form a rotating circulation. In the Northern Caspian the current regime is determined by river runoffs and prevailing winds. Here, especially in summertime, in shallow areas, strong winds and surges can completely change local currents. So, for example, surges can flood a coastal strand up to 30 kms broad in lowland area lying between the delta of the Volga and the river Ural.
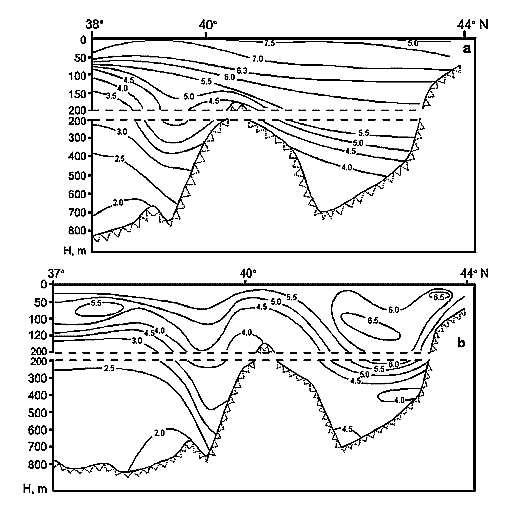
Fig. 19. Distribution of dissolved oxygen (ml/l) at the longitudinal transect: a – in February, b – in August.
The Caspian is a storm sea. From November to March of chopiness of the sea reaches force 6. The quietest period is the end of a spring and the first half of a summer. A maximum force of chopiness was registered at western coast of the Middle Caspian. Waves with the height of 10 ì and the length of 100-150 m were observed here. The velocity of surface current along the western coast of the Middle Caspian can reach up to 20 cm/sec (Zenkevich, 1963). In the vicinity of shoals of the Northern Caspian the main mass of waters of the Middle Caspian, moving from the south along eastern coast turns to the west and embracing a mass of affluents from the Northern Caspian, moves southward along the western shore again. In southern extent of the Southern Caspian an independent rotating current is isolated. At the altitude of the Absheron crest, which separates the depression of the Middle Caspian from the Southern, a part of the current flowing southward is divided into two and crosses from western shore to eastern one. One part of the water of the Volga flow southward along western shore, and another part flows eastward, where it sets up two anticyclonic currents. Vertical movements of the Caspian waters are as well expressed as horizontal. It is ensured due to small differences in the density of surface and deep waters, and also due to winter cooling, surges, warming of deep waters owing to isentropic processes, and as a result of turbulent intermixing. Due to this the deep waters of the Caspian don’t have a dead zone (oxygen deficiency causing deaths of hydrobionts) and are extremely rich with dissolved oxygen (Fig. 19). In the deep-water depression of the Southern Caspian, the water is quite well saturated with oxygen the whole year round. Its concentration makes 2 ml/l, which is only 2-3 times less, than at the depth of 25 m. In the deep-water depression of the Middle Caspian, the concentration of oxygen is even higher and the whole year round does not fall below 4-4.5 ml/l. On the surface of the Caspian Sea in summer the amount of oxygen is close to saturation; in the Middle Caspian it is equal to 98 %, and in the Southern - 94 %, in the Northern Caspian about 90%. In wintertime, oversaturation of up to 103-105% is observed all over the Caspian.
Three main forms are clearly distinguished in the relief of the bottom of the Caspian: shelf, continental slope, bed of deep-water depressions. The shelf stretches from the coastline up to depths of about 100 m. The continental slope begins from the depth of 100 ì and stretches in the Middle Caspian up to the depth of 500-600 ì, and in the Southern up to the depth of 700-750 m. Two deep-water depressions are distinguished in the Caspian Sea. In the Middle Caspian, this is the Derbent depression with the maximum depth of 790 ì, and in the Southern – the southern Caspian depression with the maximum depth of 1025 m.
The shores of the Caspian are mainly made of Quaternary deposits. The shore of the Northern Caspian is low-lying and gently sloping, and is often heavily rugged. In the Middle Caspian, western coast is mountainous, and eastern – is elevated. In the Southern Caspian, the shore is mountainous and rugged.
Fig. 20. Transparency of the water (m) in the Northern Caspian Sea: April-May, June-July, August-September, October-November.
Fig. 21. Transparency of the water (m) in the Caspian Sea: February, April, August, November.
The waters of the Caspian Sea are characterized by high transparency (Fig. 20, 21). The most transparent are the open waters of the Southern Caspian. Transparency of this part of the Caspian measured using the disk of Sekki (a white disk for measurement of water transparency) during the whole year makes 15 ì, and in the end of summer, for example, in August, can be even 20 m. In the Middle Caspian the transparency of open waters is a little bit lower, it is about 10 ì the whole year round and only in summer can reach 15 m. In the Northern Caspian, because of large inflow river drifts the transparency is very low and is usual less than 1 ì, and only at a great distance from the deltas of the Volga and Ural the transparency increases up to 7-8 m. However, bar river suspended drifts, the shallowness of the Northern Caspian also contribute to the low transparency of waters of this area. In storm weather, strong wind pushes the water to the bottom and lifts sediments. In these storm conditions, the transparency of the waters the Northern Caspian is almost completely lost. The maximum transparency of waters of this area is observed under ice cover in wintertime.
There are 11 big gulfs in the Caspian Sea: Komsomolets, Mangyshlak, Kazakh, Kara-Bogaz-Gol, Krasnovodsk, Turkmen, Kyzyl-Agaj, Agrakhan, Kizlar, Gorgan and Enzely. There are also a lot of islands. Their total area is 350 êì2. Of these the largest are - Tyleniy, Chechen, Artem, Ogurchin, and Zhiloy.
The trophic level and primary production of the Caspian Sea is low. Majority of nutrients brought in the Caspian with waters of the tributaries and, first of all, the Volga. Initially, nutrients used to come into the Caspian mainly in late spring or early summer. However, when the rivers became regulated (mainly the Volga), numerous dams detain and include in circulation of river reservoirs a part of nutrients (dissolved and suspended) and it leads to a sharp decrease in the amount of phosphorus and silicon coming into the Caspian at present.
Huge extents of the deltas are covered with macrophyte thickets. For example, 10 thousands êì2 of the delta the Volga is covered with macrophytes. These macrophytes detain nutrients running into the Caspian. Up to 70 % of dissolved inorganic phosphorus and 50 % of dissolved inorganic nitrogen is accumulated here.
Levels of nutrients in the Caspian Sea are low, even in the Northern Caspian, which is the most productive and rich part of the sea. Eutrophic conditions are observed only in some regions adjacent to the delta of the Volga. Levels of nutrients in the Middle and Southern Caspian are very low. In these parts of the Caspian Sea the nutrients arrive at the expense of an internal recirculation and with small runoff of rivers flowing into the Middle Caspian such as Terek, Sulak and Samur, and the rivers of the Southern Caspian such as Kura, Sefidrud and Atrek, and also with rains. It is possible to assume that salt and dust storms coming from the former bottom of the Aral Sea could transfer by air (together with wind) into the Caspian more nutrients at present, than before desiccation of the Aral.
Thus, it is incorrect to name the Caspian a rich lake with high productiveness. The Caspian Sea is a poor lake in terms of production, only the Northern Caspian is not so poor.
Due to diversity of conditions existing in different parts of the Caspian Sea, certain of water areas and the coastal zone serve as habitats for certain groups of organisms.
Fishes have three main habitats through their life: spawning ground, feeding ground and wintering ground. For freshwater fishes (fishes making short migrations) these areas overlap. Semi-anadromous fish species feed in the sea and return to rivers only for wintering and spawning. Anadromous species live in the sea, return to rivers only for spawning. The pelagic species spend their whole life in the sea. Some of them migrate to considerable distances within the sea, others spend their life on a rather small area. Majority of Caspian fishes range in coastal zones at the depths of 50-75 m.
Semi-anadromous fishes spawn in lower stretches and deltas of the rivers. Therefore lower stretches and the deltas practically of all rivers are of high value as spawning grounds. Semi-anadromous fishes feed in areas of the sea with fresher waters. For majority of commercial species, the optimum salinity is up to 8 gr/l. During high water years, such an area with low salinity embraces the entire Northern Caspian and estuaries of the rivers of the Middle Caspian. Semi-anadromous fishes winter in lower stretches of the rivers, deltas or estuaries. In the Northern Caspian, semi-anadromous fishes give preference to the estuary of the Ural, Volga and Terek, though they feed in all parts of the area.
Fries of anadromous and semi-anadromous fishes are fattening in the cis-Kura region, Kyzyl-Agaj gulf and Lenkoran coast of the Azerbaijan sector of the Caspian. Besides, brood stocks of anadromous and semi-anadromous fishes gather in this area of the Southern Caspian, in front of the mouth of the Kura, ready for spawning. This region becomes especially important during vernal-estival period and, to less extent, in autumnal period.
At the territory of Turkmenistan, semi-anadromous fishes spawn in the Atrek. Damming of this river at the territory of Iran had led to loss spawning grounds of semi-anadromous fishes, such as zander (Lucioperca lucioperca), carp (Cyprinus carpio), Caspian roach (Rutilus caspius) and even sturgeons.
Sturgeons are the most valuable fishes, which are spawning in the rivers of the Caspian basin. They can run upstream for hundreds kilometers. Sturgeons prefer pebbly and solid sandy ground select for spawning. Damming of the lower extents of the rivers (except for the Ural) has restricted access of sturgeons to spawning ground and sharply reduced the number of accessible spawning grounds, which had adversely affected on the extent of natural reproduction.
Nowadays, sturgeons spawn in the Ural at the distance of up to 800 kms from the mouth (mainly on sites located 250-700 kms off the outlet). Owing to siltation, the total area of spawning grounds has plummeted from 1700 ha in the end of the 1970s up to 1100 ha in the beginning of the 1990-s and is still reducing (Bokova, 1992).
In the Volga, sturgeons spawn only at the stretches down of Volgograd. As a result of damming only 372 ha of available spawning grounds remained from the previous existed spawning grounds of sturgeons of 3390 ha (Zonn, 1999).
Natural spawning grounds of sturgeons in the Terek are located in the middle of the river channel: lack of fishways through the Karhaline hydrosystem sharply reduces available spawning grounds. The river Sulak is dammed with a cascade of reservoirs. Spawning grounds with pebbled ground, mostly inundated, are located at the lower stretch of the river with length of about 100 kms. At present the total available spawning grounds of the basins of these rivers makes 20 ha.
Five fluvial spawning grounds with the total area of 112.8 ha now remain of 53 sturgeon spawning grounds in the Kura and 290 ha in the Araks (Voinova, Alekperov, 1992).
The role of the Iranian rivers for spawning of sturgeons is insignificant.
Feeding migration of sturgeons occurs all over the Northern Caspian, at western and eastern coast of the Middle and Southern Caspian at the depths of up to 50 m. In wintertime, they mainly live at western coast the Middle Caspian and at the shelf of the Southern Caspian. Considerable shoals of sturgeons are concentrated on pastures, at the extent from the southern end of the Cheleken peninsula up to the border with Iran.
Anadromous herrings used to spawn, mainly, in the Volga. After damming of the Volga, they have appeared be cut off from the main spawning grounds, and their population has considerably dwindled. Nowadays, only the black-backed shad is of commercial importance, whose spawning grounds are located in the lower stretches of the Volga.
The range of commercial marine species of fishes includes the entire sea, but with quite significant differences. The shallow northern part of the sea is of high importance as a region of reproduction of marine species, development on early stages of their life and a feeding ground of adult fishes. The common sprat, Dolginka shad, Caspian shad and big-eyed shad refer to species reproducing in the Northern Caspian.
The Caspian marine species don’t have a clear wintering period, which is attributed to favorable climatic conditions ensuring the whole year round development of fodder organisms and active feeding of fishes. The Middle and Southern Caspian play an important role for them as a feeding ground.
In the Azerbaijan sector of the Caspian, the Cis-Kura region, Kyzyl-Agaj gulf and Lenkoran coast play a determining role in shaping the ichthyofauna of the Southern Caspian. Silty-sandy, sandy-silty and silty-shelly grounds, which are considered as the most populated with benthic fodder organisms, occur in this part of the sea at the depths of 10-50ì. Therefore, fries of anadromous and semi-anadromous fishes gather for fattening in this region. Besides, brood stocks of anadromous and semi-anadromous fishes, ready for reproduction, also gather in this region of the Southern Caspian. This region becomes of especially high importance during vernal-estival period and, in a lesser extent, in autumnal period. Herrings and sprats also come to this region for reproduction, and also to the coast of the Middle Caspian and Yalama-Divichy zone at the depths of 10-50 ì; fries of sturgeons gather here for feeding in vernal period. Wintering and feeding grounds of sturgeons on marine pastures at the depthes of 10-40 ì are located in the regions of western coast of the Middle and Southern Caspian. Therefore the whole western coast of the Middle and Southern Caspian can be considered as a sensitive fish habitats.
Usual wintering grounds of several species of sprats (mainly, commercial anchovy and big-eyed sprats) are located at Turkmen coast, around Zdanov’s and Livanov’s banks and to north at the sea at the latitude of Kianly and Jafara. The big commercial stocks of valuable species herrings and both species of mullets occur in the open sea of Turkmenistan from island Ogurchin to Chikishlar. 26 fish species range in the Turkmenbashi gulf, of these gobies, sandsmelts, herrings, mullets, pipe-fish and Caspian roach are the most abundant, and Caspian inconnu, Caspian trout, Russian sturgeon and stellate sturgeon are rare.
There are feeding areas for fries of herrings, mullets at northwestern extent of the Turkmenbashi gulf. Recently, mass entering the Caspian roach, carp (with attempt to spawn), and fries of marine zander into the gulf has been noticed. The composition of the ichthyofauna of the North-Cheleken gulf is poorer because of greater salinity. Species of Mediterranean origins (mullet, sandsmelt, goby, and herring) predominate in this gulf. A false spawning run of the Caspian roach (autumn) was noted.
Some 100 rivers flow into the Caspian in Iran, which are of local importance for fish reproduction. The rivers Sefidrud, Garesu, Gorganrad, the gulf Gorgan, lagoon Anzaly, Amirkilayah, and Astara and other are of the greatest importance.
Major bird flyways, of particular importance in the context of bird migration in general in Eurasia, lie in the region of the Caspian Sea (Dolgushnik, 1960-1974; Belik, 1996; Birds of the USSR, 1987, 1988; Fauna of the USSR. Birds, 1961, 1962; Birds of the Soviet Union, 1952). In the same time, major migratory streams goes from the Siberian-Asian part of the continent. In autumn, birds are concentrated at northeastern and northern coasts of the Caspian, then, along western shore they fly southward, where this stream is divided: a part of birds continues its migration along western coast of the sea to the south, and another part migrates westward to Caucasus. Birds from central regions of Russia and partly from the northwest migrate to the coast along the Volga. In the region of the delta of the Volga this stream merges with a stream of migrants from an Asian part. Channels of the rivers Ural and Emba are important migratory routes. Vernal migrations pass in the opposite direction.
Birds’ migration occurs both in daytime, and in nighttime. The average height of night flights of passerines 710 ì, and waterfowls and shore birds - 1100 ì, and the minimum heights are more than 100 ì, which is quite important during migrations over high structures (pipes, power lines). There are no more than a score of bird species (mostly seagulls) with extremely low number are registered over deep-water stretches of the sea removed from the shore at the distance of 10 kms and more. Individual representatives of passerines, birds of prey, and sterns are also observed.
The main mass of migrants of this group, both flying over the area and feeding aggregations, sticks to a narrow coastal band within 1-2 kms. The main concentration of birds is observed in gulfs with the depths of 0.5-1ì along the edge of a reed belt, where silty and sandy bottom is overgrown with surface vegetation. The number of birds is lower at sandy denuded stretches of the coastline. Mostly seagulls, stints and some other birds occur here.
Except for birds of wetlands, inhabitants of forests and bushlands are found in the coastal zone during migration (thrush, warbler, finch and bunting), and also birds of open deserted and steppe landscapes (lark, pipit, wagtail, wheater etc.). Reed thickets and also accompanying them silty banks and shoals play an important role for migratory birds as a stopover for feeding, resting, waiting for a weather window (often storms, cold weather) and recovery.
The reed belt with open reaches of water and a coastal strand up to 1 km across is an important place for nesting and migratory aggregations of birds, including valuable hunting species and species listed in the Red Book such as heron, pelican, flamingo.
During stopovers, the birds are concentrated in regions of deltas of large rivers such as Emba, Ural, Volga, Kuma, Terek and Samur.
Representative features of settled and partially settled fish-eating species include seasonal roamings and uneven distribution along the coast. In spring pelican and cormorants, wintering in Lenkoran and Iran, move northward along western and eastern coasts. The concentration and nesting of birds is observed in the region of the Agrakhan gulf, in regions of deltas and along the channels of the rivers Samur, Bulak-Chay, Sulak and Terek (Dagestan) and at southern extremity of Mangyshlak, and in the north in the delta of the Volga and partly of the Ural.
The flock of cormorants wintering in Dagestan (Pakhulskiy, 1951) ranges in waters of this republic from November to June. In summer, in July, a considerable part of the population of cormorants moves from western coast for feeding to the region of the delta of the Volga, where it stays till October, and from eastern coast– to the coastal region between the Ural and Emba (between the mouth of the Ural and the spit Zhiloy). In autumn, a part of these birds flies westward, where they mix up with the population of the Volga delta, and another part migrates to southern part of the Caspian and Iran. From the delta of the Volga, the autumnal migration moves along western coast. They fly along the Dagestan coast in the end of October - beginning of November.
In general, we should note that some 80 % of cormorants and herons, inhabiting the Northern Caspian, are concentrated in the region of the Astrakhan reserve.
In summertime (including molting aggregations in post-nesting period) and during migration, waterfowls (ducks, geese and coots) are concentrated along the coast of the Northern Caspian. Bird concentrations in the regions of the deltas of the Volga and Ural, coast between the Volga and Ural, the spit Zhilaya and along eastern coast of the gulf Komsomolets (Kazakhstan) are especially great. At western coast, bird concentrations are found in deltas of large rivers (Terek, Sulak), in Agrakhan and Kizlar gulfs. In autumn, in eastern sector, birds are concentrated along the peninsula Mangyshlak.
In northern cis-Caspian, geese are especially abundant during autumnal transit migration, which occurs in October - November. The magnitude of migrating birds flying in southwestern direction reaches 100-200 thousands (Belik, 1996). In the same time, of these 60-90 % are white-fronted geese (Anser albifrons), 10 to 40 % - greylag geese. The population of migrating lesser white-fronted geese does not exceed, probably, 1-2 thousand birds. Bean geese are extremely rare.
The main flyway of stints in eastern sector lies from the spit Zhiloy to the peninsula Buzachy. During the nesting period, big concentrations of seagulls were observed on the islands in the vicinity of the peninsula Mangyshlak (Kulaly, Morskoy, Rybachiy, etc.) and on islands of western coast (Chechen, Tuleniy, etc.), but after inundation of majority "shalyg" nesting occurs mainly in coastal reeds.
Molting of many waterfowl species pass during the post-nesting period, when they are completely unable to flight for several weeks. In the Northern Caspian, it happens in July - August. In this period, birds are extremely vulnerable and are concentrated in certain difficult places. For instance, swans (Cygnus), ducks (diving and river), coots (Fulica atra) are gathered at the external boundary of a reed belt at northern coast and in the delta of the Volga. In the same time, the local population is supplemented with a considerable number of birds, which nested north of the Caspian. The flamingo (Phoenicopterus roseus) moults at the territory of Kazakhstan, in the gulf Komsomolets.
The main wintering grounds on the Caspian are located in its central and, to a greater extent, in southern part. However, some species winter at the peninsula Mangyshlak and in the south of Dagestan (delta of Samur). The group of birds wintering here includes mainly species of diving ducks, swans (mainly, the mute swan (Cygnus olor)) and number of other species, such as cormorant (Phalacrocorax carbo). There is a possibility of wintering of significant numbers of the Bewick’s swan (Cygnus brewickii) in the Northern Caspian at the edge of the ice cover (Morozov, 1996).
278 species of birds are fount at the coasts of Kazakhstan, of these more than 240 species occur in lower stretches of the Ural. In the 60-s, 84 species of birds used to nest at the coast of the Caspian from the delta of the Volga up to the mouth of the Emba, of these 41 species were most representative for coastal reed-cat’s tail thickets, and 13 species for sandy islands and banks. Nowadays, up to 70 bird species nest in the delta of the Ural. The core of the ornithofauna is made of representatives of the wetland complex (40 species). The delta of the Ural and adjacent shoals are of huge significance as a place of estival molting concentrations of river and diving ducks, gray geese and mute swans. If in the 50-70-s, the major stopovers used to be kultuks (deeply jagged shallow bays) and marine shoals, then at present, new internal reaches of the delta of the Ural are more preferable for river ducks, coots and partly mute swans.
The leading place by abundance of species and quantitative characteristics, after the deltas of the Volga and Ural, takes the floodplain of the Emba. Both coastal inhabitants, and typical inhabitants of deserts (including rare species listed in the Red Book of the Republic of Kazakhstan - bustards, sandgrouses, cranes, birds of prey etc.) are found here.
An increase of sea levels, flooding and resulting extension of reed thickets and other surface vegetation has led to significant alterations of ranges of many shore birds. Along with an increase on nesting grounds of the populations of ducks, swans and herons, and emergence of new species (purple gallinule and cattle egret) on nesting ground, it was noted that they are moving to nesting grounds on recently flooded areas, closer to the present coastline.
The inundation of shell islands (shalyga) in northeastern part of the Caspian and emergence of reed thickets expands the areas suitable for nesting of many bird species. These areas are actively populated by the mute swan, pochard, coot, seagull and tern. Until recently, majority of these birds have occurred on these areas only during migrations. On shell islands, in the region of Karaton and the mouth of the Emba, on 2 - 3 June 1996 we meet 4 pairs of mute swans with broods and 4-6 nestlings. The herring gulls are used various structures for nests. Three colonies of herring gulls on ship wrecks, 70-100 pairs in each, with nestlings of different age, were found on the peninsula Buzachy, on 25-26 May.
Peer review of the population of waterfowls and shore birds at northern coast of the Caspian from the Kazakhstan part of the delta of the Volga to the mouth of the Emba (in comparison with 1992-1993) was almost the same. In the coastal zone from the mouth of the Emba up to the peninsula Buzachy, presently nests not less than 100 pairs of mute swans, up to 500 pairs of red-crested pochards and coots, and up to 400-450 pairs of herring gulls. In small numbers, white egrets and gray herons are found in small numbers. Besides, little egrets and spoonbills are found here during the nesting period.
The border of the regular wintering zone of waterfowls in the Kazakhstan part of the Caspian Sea passes through the Mangyshlak gulf. In soft winters, up to 3 thousand ducks and swans winters at the estuary of the Volga and Ural. A new center of wintering has formed in the vicinity of Aktau and Karakol Lake, where up to 20 thousand of swans wintered in certain years, during the last decade.
More southerly regions of the Caspian do not freeze in wintertime and serve as a wintering ground for migratory birds. These are Azerbaijan, Iranian and Turkmenistan coasts.
In Lenkoran region of Azerbaijan are present 3 such as wetlands:
The reservoirs of southeastern part of the Kura - Araks lowland and open coastal shoals, located on its border, are rich and diverse as a habitat of waterfowls and shore birds. The Kyzyl-Agaj and Shirvan reserves and the Bandovan sanctuary are located here. In general, the above area, as habitats of waterfowls and shorebirds are subdivided into 8 types of lands:
There are 4 main wetlands at the Absheron-Gobustan coast of the Caspian Sea:
Three main wetlands exist at the Samur-Divichy coast of the Caspian Sea:
The most sensitive habitats of waterfowls and shore birds in the Azerbaijan sector of the Caspian coast are the water bodies of the Kyzyl-Agaj reserve (Major and Minor Kyzyl-Agaj gulfs, Pirman lagoon, Akushin and Kharaz floods), delta of the Kura, Puta-Pirsahat coast (islands of the Baku archipelago), the coast of the Shakh spit and lake Akh-Zybir.
A great number of waterfowls and shore birds accumulate at water bodies of the Kyzyl-Agaj reserve, in the delta of the Kura and at the Absheron-Gobustan coast during migration and wintering. A unique colony of copepods and waders nests in the Kyzyl-Agaj reserve and in the delta of the Kura every year. Seagulls and some species of stints nest at the Puta-Pirsahat coast, on islands of the Baku archipelago and at the Shakh spit.
Lakes Akh-Zybir is a large stopover during migration of waterfowls.
The Turkmenistan coast of the Caspian due to its climatic features is an important region of bird wintering and migration. The most important habitats are Turkmenbashi, North-Cheleken, Balkhan and Mikhaylovskiy gulfs.
The Balkhan gulf is the second largest of the above gulfs. It has increased almost by the factor of 7 from the beginning of the transgression, by filling the bed of the ancient Aktama (neighboring area of Western Uzboy). Predominance of shoals, good insulation and plenty of fertile silt covering its bottom caused vigorous development of underwater macro- and microphytes and zoobenthos. Sufficient protection and rich fodder basis (more than 15 species of zoobenthos, 59 species of underwater macrophytes, diatoms) allowed this gulf to become the main ground of mass wintering of waterfowls and shore birds (some 44 % of their total population), and mass nesting ground of colonial species, including Larus ichthyaetus, listed in the Red Book. The density of wintering birds has increased by a factor of 30 in comparison with 1984, making this gulf the main place of concentration of valuable and rare species of ornithofauna of Turkmenistan.
The main natural feature of the system of gulfs "Turkmenbashi and North-Cheleken" is position of under their bed of a thick layer of arboreal of deposits brought here by ancient Uzboy. That is why as distinct from other, not less extensive, marine gulfs of eastern coast of the Caspian Sea, there is such abundant and diverse zoo- and phytoplankton, zoo- and phytobenthos, underwater higher flowering plants and fauna of vertebrate animals. The biodiversity of the fauna and flora of these wetlands includes about 600 species, of these 30 are included into the Red Book of Turkmenistan; a considerable part them are endemic species of the Caspian.
Another quite important feature of the above wetlands is at that they are, with certain exceptions, included into the structure of the Khazar reserve. Numerous legal instruments of Turkmenistan and practical work of personnel of the reserve are the security of preservation of its natural complex in natural conditions. Due to unique features of this natural complex, the status of wetlands of international importance awarded to the above system of gulfs (Ramsar Convention, 1971).
Recognizing basic importance of the above described system of gulfs for the environment of southeastern Turkmen coast of the Caspian Sea, nevertheless, the other areas located north of and south of it, representing numerous marine shoals, small gulfs and kultuks, straits and bays, and also lower stretches of the river Atrek (altogether about 30 sites) make a significant part in the structure of wetlands of southeastern Caspian and play a noticeable role in shaping their biotic communities.
The comparison of wintering at all 3 wetlands of southeastern coast of the Caspian (Northern, Central and Southern) has shown that, despite of a predominating role of the central coastal area (Marvous wetlands), reservoirs of its remaining parts attract in average more than 30 % of the whole population of birds with the specific diversity of 65 % for northern and 78 % - for southern areas.
An analysis of long-term (1972-1996) dynamics of the magnitude of winterings of waterfowls and shore birds at these wetlands has shown that irrespectively of the condition of natural components of ecological situation, the importance of wetlands of northern and southern areas is slowly and steadily increasing. Probably, this is facilitated by a number of natural factors developing in accordance with the 20-year transgression of the Caspian Sea.
The deltas of the rivers of gulfs and lagoons of Iranian coast of the Southern Caspian are favorable for wintering of birds.
Only seals range in the Caspian Sea of marine mammals in. In wintertime, seals are concentrated in the Northern Caspian at the edge of the ice cover. Their pupping, mating and molting happens on ice. A small group of seals stays to winter on islands at the coast of Turkmenistan. In summertime, seals migrate to the Middle and Southern Caspian for fattening. A certain part of the herd remains in the Northern Caspian.
Coastal mammals, connected to the sea, inhabit mainly in the coastal strand of bushes and reed thickets. These are wild boar, otter, European mink, coon, muskrat and others. The diversity of mammals, living near the shore, but preferring semi-desert and steppe biotopes, is considerably higher. Rodents, hares and a number of predatory species prefer these biotopes. Such animals as the saiga or goitred gazelle range in desert and semi-desert biotopes. They are not linked to the sea, but live in the contact zone. Three herds of saiga (Saiga tatarica) inhabit northern cis-Caspian: Usturt (between the Caspian and Aral), Guryev (interfluve of the Volga and Ural), Kalmyk herd (Kalmykia). After imposition the ban for hunting the saiga (1991), its population has reached 300 thousand animals. A lowered sheep breeding positively influenced the increase of saiga population. In Kalmykia, calving grounds are located in the region of Black Lands. Regular seasonal migrations are typical for the saiga. In Kalmykia, this means roaming within 300-500 kms. However, recently, there have been no apparent migrations. The main population sticks to the areas south of the highway Astrakhan - Elista almost the whole year round. Only the herd, ranging between the Volga and Ural, migrate to the coast in wintertime.
The goitred gazelle inhabits at Azerbaijan, Kazakhstan and Turkmenistan coasts. It is an inhabitant of deserts with solid or sandy ground fixed by routes of plants. It is listed in the Red books of all three countries.
The Caspian Sea is largest lake in the world. Based on this one can assume that it has the highest biodiversity. It is logical to assume that the more is the area of a lake, the more plant and animal organisms live in it. In our opinion this correlation with the area is rather indirect. Certainly, the more is the area of a water body, the more is the probability that it will contain more species of hydrobionts. However, there are no doubts that, in this case, the defining parameters are the age a lake, its biotope diversity, biotic and àbiotic conditions, availability of necessary and balanced amount of matters and energy.
An important feature of the Caspian is the extreme diversity of biotopes, biotic and àbiotic conditions (Zenkevich, 1963). First of all, water salinity in different parts of this lake is quite different (Kosarev, Tuzhilkin, 1995). In certain areas, especially those neighboring with deltas of rivers, the water is almost fresh. In other places, and, first of all, in shallow gulfs of eastern coast (the gulf Kara-Bogaz-Gol) the water is heavily mineralized and often even hyperhaline, with lake salt. In open regions of the Northern Caspian, at the transect from the north to the south, the water salinity smoothly increases from freshwater (in deltas of the rivers Volga and Ural) to 10-12 gr/l (at the border of the Northern and Middle Caspian). At the transect from the east to the west drops sharply - from 60-40 gr/l on eastern shoals of Kazakhstan, up to 6-7 gr/l in the center of the Northern Caspian, and up to fresh water at western coast, in estuarine regions of the river Terek.
The average salinity of the Northern Caspian is 5-8 gr/l, and this area of the Caspian, based on the Venetian classification of water salinity, should be referred to oligo-mesohaline water bodies. In open regions of the Middle and Southern Caspian, salinity is more stable in comparison with the Northern Caspian and ranges from 11 up to 14 gr/l. Only in areas adjacent to deltas of rivers, it becomes much lower. The average salinity of the Middle Caspian is 11-12 gr/l, and the Southern Caspian - 12-13 gr/l. Thus, both areas of the Caspian, based on the Venetian classification, should be referred to mesohaline water bodies. The shallow gulfs of eastern coast (Kara-Bogaz-Gol, Kaidak, Komsomolets, Mertviy Kultuk etc.) have the salinity from 13-14 gr/l at the entrance into a gulf and up to lake salt at the opposite shoals of the gulf. Thus, this area of the Caspian, based on the Venetian classification, should be referred to hyperhaline water bodies.
Such variable salinity conditions strongly increase biodiversity of the Caspian. Due to this, freshwater, brackish, euryhaline and hyperhaline hydrobionts can inhabit the sea, and due to proximity of chemical compositions of marine and Caspian waters many marine organisms will perfect feel themselves here. It is especially necessary to note that actually three ecosystems coexist in parallel within the borders of the Caspian: freshwater, brackish and hyperhaline and it promotes biological diversification of this lake.
The vertical stratification of water salinity in the modern Caspian is poorly expressed and the water salinity of the sea floor hardly differs from that on the surface. Under the present very weak vertical stratification of water salinity, good intermixing of waters is observed in this lake, due to this the bottom waters are rich in oxygen. However, earlier, when the level of the Caspian was much higher than now, and there was strong vertical stratification of bottom salinity, the oxygen was practically absent at the bottom (Kosarev and Tuzhilkin, 1995; Dumont, 1998). That is why, nowadays, there is poor life at the depths of more than 100m! Presently, there is no abyssal fauna and flora in this lake. Probably, modern animals and plants "generically remember" the recent disastrous cases of deep oxygen depletion. It is possible to assume that this ancient natural ecological catastrophe heavily reduced modern diversity of the Caspian fauna and flora. If numerous ancient sea levels rises had not resulted in mass "suffocation" of deep-water inhabitants, today deep-water biodiversity of this lake would be, apparently, higher than that of Baikal.
Except for strong differences in salinity of different parts of the Caspian, the temperature parameters are also extremely diverse and it also contributes to its biodiversification. The elongation of the Caspian from the north to the south for more than 1200 kms results in strong differences of seasonal temperatures. In wintertime, when up to the half of the Northern Caspian is covered with ice, the temperature of the Southern Caspian, even during the coldest months, never lowers below + 8-90 C. Such a latitudinal stratification of surface water temperatures allows both cold water, and warm water hydrobionts to live in this lake. Besides, there is a strong vertical temperature stratification in the Caspian. After the zone of a temperature jump, the water temperature remain low even during the hottest summer months. Seasonally independent thermostatic cold water conditions are actually observed in deep water parts of this lake. The presence deep cold water in the Caspian allows populating its upper horizons even with arctic organisms (Zenkevich, 1963; Kosarev, Yablonskaya, 1996). As well as in case of varying salinity, the diversity of temperature conditions of the Caspian increases its biodiversity. It is possible to state that there are three types of water bodies within the Caspian: cold, moderate and warm.
Production characteristics of various areas of the Caspian are also quite different and, as well as in case of salinity and temperature, this results in magnification of biodiversity of this lake.
Production characteristics of the Caspian Sea are determined by receipt allochthonous organic matter, mainly, with river runoffs and eolian precipitation; autochthonous organic matter, i.e. production of organic matter by phytoplankton and higher water plants, development of bacterial biomass and destruction of organic matter in water and ground. M.A. Salmanov (1987) presented the most complete information on production processes in recent years. In compliance with his calculations, the ratio between the total production of phytoplankton and bacterioplankton makes 1:0.45. Gross production of primary organic matter (OM) of phytoplankton for the period of 1964-1984 reached 143.4m tons of carbon. In the same time, in northern, middle and southern parts of the Caspian formed, accordingly, 19.9, 44.4 and 35.77 % of organic matter. It is necessary to note a very high producing capacity of the Northern Caspian, as it makes 0.5 % of the total volume of the sea, and produces about 20 % of organic matter. It is quite typical that the areas of high production in the Northern Caspian mainly belong to landscape ecotones, both by bottom topography, and gradient of salinity variations. Undoubtedly that the mineral elements arriving with river runoffs serve as a basis for biological production. The areas of the Caspian adjoining to river estuaries are always characterized by increased producing capacity due to organic matter brought by rivers. For example, due to large runoff of the Volga and Ural, the entire Northern Caspian is considerably more productive, than the Middle and Southern Caspian. The certain regions of eastern coast of the Middle Caspian are also distinguished by an increased producing capacity due to upwelling. Thus, hydrobionts both inhabiting in oligo- and mesotrophic water bodies, and inhabiting in eutrophic ones can find a suitable habitat in Caspian (Karpevich, 1975).
The sea levels rise up to -27.75ì lead to an increase of the absolute production of OM in the Northern Caspian due to extension of shallow areas, where an active photosynthesis occurs. Calculations show that annual production of OM in the Northern Caspian has increased by a factor of 1.68, and now makes 47.720m tons. The percentage relation between the parts of the sea from the north to the south has changed as follows - 29.3, 39.2 and 31.5%. If we had transferred the annual production from carbon to raw phytoplankton material, it would make 280.6m Ò.
The modern fauna and flora of the present Caspian Sea consists of the four main components: 1 – of Caspian origins; 2 – of arctic origins; 3 – of Atlantic and Mediterranean origins; 4 – of freshwater origins (Derzhavin, 1912; Knipovich, 1938; Berg, 1928; Zenkevich, 1963).
In Zenkevich’s opinion (1963), the fauna and flora of the Caspian Sea usually could not compete with invaders and often such invaded fauna and flora destroyed native species.
The biodiversity of the Caspian Sea is 2.5 times poorer, than that of the Black sea, or 5 times poorer, than that of the Barents sea (Zenkevich, 1963). The main reason of this is probably its variable salinity. For the present freshwater fauna and flora, the water salinity is too high, and for the present marine species - low. Thus, the modern Caspian Sea is a real paradise for brackish water species originating both from marine, and from continental water bodies (Birstein, 1939; Mordukhai-Boltovskoy, 1979). However, in our opinion, the comparison with purely freshwater lakes shows that unstable salinity of the Caspian is more favorable for diversification than for loss of biodiversity.
The phytoplankton of the Northern Caspian is different from that of the Middle and Southern Caspian with typical features of estuarine plankton, impoverished by marine elements (Proshkina-Lavrenko, Makarova, 1968). The phytoplankton of the Northern Caspian in 1986-1994 consisted of 230 species, the Middle and Southern - 82 and 83 species, correspondingly. According to data of the latest reports specific composition of plankton microalgae only of the Northern Caspian (Hydrometeorology …, 1996) includes more than 400 species (Cyanophyta - 90, Chrysophyta - 1, Bacillariophyta - 149, Pyrrophyta - 58 Euglenophyta - 7, Chlorophyta - 138). However, despite of this diversity, only a few species are predominating. In relation to salinity, algae are subdivided into 5 ecological groups: freshwater, brackish and brackish-freshwater species, and a small number of marine species and halophobes (Levshakova, 1971).
Of freshwater algae, the green algae are on the first place by the number of species. On shallow areas, filamentous algae of genera Zygnema and, especially, Spirogyra have the highest biomass. Blue-green algae consist of freshwater and brackish-freshwater species. There are also marine and brackish forms, but their role is insignificant. Diatoms are widespread all over the Caspian and are equally diverse in all ecological groups. They take a leading place by number of species; their specific composition is the most stable during the whole vegetation period. A large marine alga, Rhizosolenia calcar-avis takes the leading place by biomass. Perydine algae are represented by mainly marine and brackish water forms and are of high importance in the trophicity of the sea. A special role belongs to Exuviaella cordata, which dominates by numbers and is inferior to Rh. Calcar-avis by biomass, only because its cells are ten times smaller. The specific diversity decreases southward due to shedding of freshwater forms. The share of marine species increases from 7 % in northern part of the sea up to 27 % in more southern regions.
A marine diatom, Rhizosolenia calcar-avis makes the basic part of the phytoplankton of the Middle and Southern Caspian. At present, its amount remains the same in the Middle Caspian, but has highly increased in general. In the Middle Caspian, in the 90-s, eastern region was the richest by the number of phytoplankton species and their population.
Long-term changes in development of phytoplankton of the Northern Caspian are naturally connected to the amount of nutrients brought in by the Volga. The dependence of biomass of diatoms on the amount of silicon, and the whole primary production of a community - on phosphorus brought in by freshets has been established. Due to damming of the Volga, the role of diatoms has decreased in regards to a period of the natural regime, though in the same time, the share of rhizosolenia has increased, and a leading position was taken by green algae, particularly Spirogyra, while the species of brackish water and brackish-freshwater complexes had (mid of the 1970s) a minimal development. In the modern period this algae are rare and now intensive development of small colonial species of blue-green and green algae has an effect for the aggregate number of phytoplankton. The concentration of phytoplankton in western half of the Northern Caspian is higher, than in eastern. The species of freshwater and brackish water complexes are predominating. Diatoms are most ubiquitous. Blue-green and green algae range in shallow regions and, mainly, the estuary of the Volga. Pyrophytes occupy mainly deep areas of the sea, where the water salinity is higher. The yellow-green alga, Dinobrion sertularia, used to be found at outlets of fishways in certain years. Euglenas are found only in regions with fresher water. As distinct from the previous low water period, species, which used to be found in the 1950-1960s (Anabaena wariabilus Kutz, A. Solitaria Kleb) were again found in phytoplankton in 1986-1994. The predominating species include Thalassiosira inserta and Stephanodiscus astrae.
155 species and varieties of algae were revealed in eastern part of the Northern Caspian in the second half of the 90s: diatoms - 107 species and varieties, blue-green - 22, green - 15, pyrophyte - 8, Euglenas - 2 and yellow-green - 1. The most diverse diatoms species are Nitzchia - 20 varieties, Navicula - 14 and Cymbella – 8. Of blue-green algae - Gloeocapsa and Merismopedia - on 3. Of green algae - species Scenedesmus - 4 species.
Predominating species of vernal pelagic phytocenosis were diatoms Chaetoceros wighamii or Rhizosolenia calcar-avis with accompanying green alga Binuclearia lauterbornii or diatoms Cyclotella caspia v. caspia. The blue-green algae Aphanizomenon flos-aquae or Aphanothece clathrata and Merismopedia tenuissima accordingly predominated in coastal regions in spring and summer. Small-sized diatoms such as Diatoma elongatum, Synedra ulna, Mastogloia Smithii, Navicula cryptocephala developed in mass on shoals in summer. September plankton was characterized by ubiquitous distribution of Rh. Calcar-avis, to which in pelagialia joined Coscinodiscus Jonesianus. In October, these leaders were supplanted by Thalassiosira inserta and B. Lauterbornii. In pelagic regions of the sea in the beginning of winter prospered Rh. Calcar-avis and Cyclotella meneghiniana, in the coastal zone in February other diatoms were background species: S. Tabulata, N. Cryptocephala, species ð. Nitzchia.
The phytoplankton of the Southern Caspian at the coast of Azerbaijan is represented by 171 species. A leading role belongs to diatoms, which are widespread all over this part of the sea and have the most diverse species composition (75 species of 22 genera). By specific diversity, the genus Chaetoceros - 16 species, varieties and forms is distinguished, of these 3 are endemics of the Caspian Sea. The second by the number of species is the genus Thalassiosira consisting of 11 species, varieties and forms, of which 5 are endemics. On the third place there is the genus Coscinodiscus - 8 species, varieties and forms, ensued by genera Melosira and Nitzschia – up to 6 species, varieties and forms. Of the genus Coscinodiscus, the species Ñ. jonesianus and Ñ. granii are the most widespread. It is necessary to note the marine genus Rhizosolenia with 3 species. Such species, as Actinocyclus, Sceletonema, Thalassionema though they are represented by a small number of forms, however their representatives are widely distributed in the Caspian and play an important role in the life of the sea. In the coastal zone, in the vicinity of islands of the Baku archipelago, a special role belongs to benthic-plankton and benthic species, abundantly developing in plankton - Grammatophora, Achnantes, Campylodiscus, etc.
Blue-green algae in the Southern Caspian are represented by 55 species and forms of 13 genera. The genus Oscillatoriaceae is the most diversely is represented, from which the genus Oscillatoria is distinguished by specific diversity.
As distinct from blue-green, perydine algae are indigenous inhabitants of the Caspian, though by qualitative composition they are poorer than diatoms, but are found all over the sea and are represented by 23 species, varieties and forms of 9 genera. By abundance, perydines take the second places after diatoms. Being one of the most widespread species, it is also one of the dominants from spring to autumn. Prorocentrum scutellum, Pr. Micans, Glenodinium caspicum, etc. refer to accompanying species.
In the Southern Caspian, the green algae are represented by 15 species, varieties and forms of 8 genera. Pediastrum simplex, P. Boryanum, Scenedesmus quadricanda etc. range in coastal areas with fresher water. Binuclearia lauterbonii are also widespread. Yellow-green and Euglenas algae are represented by 2 species.
Presumably, 13 Caspian endemics occur in phytoplanktonå of the sea (Proshkina-Lavrenko, Makarova, 1968), but in recent years 3 species were found - Thalassiosira inserta, Th. Caspica and P. Parvula, and of endemics of southern seas, Chaetoceros subtilis was present.
The species composition of periphyton of the Caspian amounts to some 200 species of algae (Cyanophyta - 33, Bacillariophyta - 126, Chlorophyta - 12, Phaeophyta - 8, Rhodophyta - 11).
87 species of macrophytes, relating to 5 types, 8 classes, 17 orders, 24 families and 45 genera are known in the Caspian Sea. The quantitative ratio of types of algae is represented in the table. The most diverse with representatives of the family Cladophoraceae (11), and Characeae (11) Ulvaceae (10). The most diverse genus Enteromorpha - 9. The core of the Caspian algoflora is the green alga.
38 species of macrophytes are registered in the Kazakhstan sector of the Caspian Sea, of these only 11 are widespread in the sea, the other are found in wetlands of the transient zone and estuaries. The families of Pondweeds - 6 species, Cat’s tails - 4 species, sedges - 4 species, forgbids - 3 species are the most representative. Other families are represented by 1 or 2 species. Southern reed (Phragmites australis) and narrow-leaved cat’s tail (Typha angustifolia) are dominants in communities of the transient zone, subdominants include water plantain (Alisma plantago aquatica), bur-reed (Sparganium stoloniferum), bulrush (Scirpus lacustris), pondweed (Potamogeton pectinatus, P. Perfoliatus) and duckweed (Lemna minor). Absolute dominants of communities of seawaters are Zostera minor, Myriophyllum spicatum and Ceratophyllum demersum.
Only 5 species of higher water plants are known in eastern part of the Caspian Sea. All of them belong to flowering plants: Zostera minor, Potamogeton pectinatus, Ruppia spiralis, R. Maritima and Najas marina.
Fig. 22. Faunal composition of free-living Metazoa of the Caspian Sea, %.
(Atlas …, 1968; Mordukhai-Boltovskoi, 1960, 1978).
a – In systematic groups: 1. Turbellaria, 2. Nematodes, 3. Rotatoria, 4.
Annelida, 5. Crustacea, 6. Mollusca, 7. Pisces & Cyclostomata, 8. others.
b – In faunal complexes: 1.Autochthonous, 2. Freshwater, 3. Mediterranean, 4.
Arctic.
Fishes and crustaceans have the greatest diversity in Caspian (Fig. 22). They come to 2/3 or 63 % of the total number of species. These organisms, due to very good osmoregulatory abilities, can live in a very broad range of salinity: from fresh water up to brackish, and even in more salty water, than oceanic (Zenkevich, 1963). The prevalence of these two groups of animals in the present Caspian proves that in past the salinity in this lake used swing, and only species with very good osmoregulatory abilities could survive and to provide good speciation and adaptive radiation. In past, species with insufficient osmoregulation died out because of changes in water salinity or because their speciation and adaptive radiation were suppressed or decreased owing to negative influence of varying salinity. Thus, the modern biodiversity of the Caspian Sea simply reflects a complicated history of paleo-Caspian transgressions and regressions, desalinisation and salinization!
The first good report on fauna and flora of the Caspian Sea was published in 1963 by Zenkevich. However, this scientist used a lot of data previously published in 1951 by A. Derzhavin. According to data to two authors, 718 species inhabit the Caspian: 62 species of protozoa, 397 - invertebrates, 79 - vertebrates and 170 species of parasitic organisms. So, without protozoa and parasitic organisms, A. Derzhavin and L. Zenkevich distinguished only 476 species of free living Metazoa. Of these species, some 46 % are endemics of the Caspian Sea, 66 % inhabit also in adjacent southern seas, 4.4 % are of Atlantic and Mediterranean origins and 3 % - of arctic origins.
315 species and subspecies are registered in zooplankton of the Caspian Sea (Kasymov, 1987, 1994), of these 135 species refer to infusorias (Agamaliyev, Bagirov, 1975). The main part of zooplankton is species of Caspian origins.
The species composition of zooplankton of the Northern Caspian amounts to about 200 species. Infusoria are represented most diversely (more than 70 species). Rotatoria (> 50), Cladocera (» 30) and Copepoda (> 20) are less diversely represented (Kasymov, 1997). Only 30-40 species are found quite regularly. Meroplankton, represented, mainly, by larvae of bivalves and crustaceans contributes to biodiversity of plankton communities during reproduction. Changes of complexes of organisms, from brackish to euryhaline and marine, are observed from northern border to the south.
In the 70s, under low sea levels and increased salinity, Eurytemora minor, Polyphemus exiguus, species of pp. Apagis and Cercopagis, arctic invader Limnocalanus grimaldii are widely distributed, especially, in deepwater areas. Mediterranean euryhaline and eurythermal species such as Calanipeda aquaedulcis and Pleopis (Podon) polyphemoides dwelled everywhere but concentrated in coast areas. A freshwater complex - rotifers and cladocerans, occupied shallow and estuaries with fresher water: species ðð. Brachionus, Moina, Diaphanosoma, and Bosmina.
Representative species of zooplankton in coastal shallow zone of the Middle and Southern Caspian are — Calanipeda aquaedulcis, Acartia clausi, Heterocope caspia, Podonevadne camptonux, and P. Angusta. The presence of larvae of benthic organisms in mass is representative of vernal and summer plankton of coastal zone. Both in the Middle and Southern Caspian, more than 50 % of the total biomass of plankton is formed by the larvae of Balanus in spring, and by the larvae of Mollusca in summer (Bagirov, 1989).
Eurytemora grimmi, E. Minor, Limnocalanus grimaldii and opossum shrimps - Mysis caspia, M. Macrolepis, Paramysis baeri are dominating species of the central holistic zone.
The transient zone has a mixed composition of population, species of both shallow and central holistic zone are found here. By biomass, Eurytemora grimmi and E. minor dominate here.
At western coast of the Middle and Southern Caspian, cladocerans (Cladocera) are dominating by the number of species of zooplankton, 55-25 % of the total number of species. Of copepods, the dominating species are Eurytemora grimmi, E. Minor, Acartia clausi, Calanipeda aquaedulcis, and Limnocalanus grimaldii. These species are found throughout a year. Of cladocerans, Podon polyphemoides, Podonevadne trigona, Camptonyx macronyx, P. Camptonyx podonoides, Evadne anonyx producta, E. Anonyx deflexa etc. are found here.
A stable trend of increasing of numbers of species of freshwater complex (with 54 up to 62 %) is observed in zooplankton Northern Caspian, in the modern period, due to increased discharge of the Volga, rise of sea levels and expansion of its area. In recent years, researches found 81 species. Mainly, the rotifer-cladoceran plankton predominated.
68 species and varieties were discovered in zooplankton of eastern part of the Northern Caspian: rotifers - 24, cladocerans and copepods - 17 each and others (protozoa, coelenterates, conchostracs, larvae of benthic animals - molluscs, cirripeds, higher crustaceans and polychete) - 10. The most diversified group - rotifers were represented by freshwater forms (more than 80 %), living in estuarine areas of the Ural, and only 2 marine species - S. Cecilia and the endemic T. Caspica. Euryhaline and eurythermal forms of S. Stylata and heat-loving Brachionus quadridentatus, Keratella tropica are widespread, F. Longiseta limnetica is less widespread. Asplanchna priodonta and B. Plicatilis are typical for warm seasons, and S. Cecilia - for cold periods.
Of cladocerans, a marine crustacean, brought in by water current from the Middle Caspian, Pleopis polyphemoides was a background species in spring, Podonevadne was constantly present in summertime. The endemic Cornigerius maeoticus hircus is less widespread, C. Maximowitschi and C. Bicornis are absolutely rare. The Ponto-Aral-Caspian endemic, Evadne anonyx avoids shallow areas with fresher water. The other representatives of cladocerans are freshwater crustaceans of the families Chydoridae and Bosmina longirostris.
The group of mass copepods - C. Aquaedulcis and Halicyclops sarsii was supplemented in the 90s with Acartia clausi. The facultative plankter, Harpacticoida was found everywhere, particularly, the endemic E. Concinnum. H. Caspia lowered considerably. E. Minor and Limnocalanus grimaldii are rarely found. Remaining copepods refer to freshwater species of coastal regions.
With the increase of sea levels, the number of freshwater species in zooplankton (from 54 up to 62 %) has increased. Rotatory plankton with an increase of the share of freshwater and brackish water complexes was predominating.
The biological seasonal prevalence in zooplankton is determined not only with temperature changes, but also with changes of salinity, connected to them. In spring after destruction of the ice cover and prior to entering into the sea of freshet waters, euryhaline and marine copepods dominate in plankton. Euryhaline Calanipeda aquaedulcis, Acartia clausi and Halycyclops sarsi defining the biomass of this group, winter under ice and begin to reproduce in April. The marine organisms, of which Eurytemora grimmi dominates in spring, penetrate deep into the waters of the Middle Caspian, but don’t not form considerable biomass.
In May, with increase of water temperatures, freshwater Cladocera and Rotatoria begin to develop reaching the maximum biomass in summer. A freshwater complex of organisms develops in a zone, where fresh and marine waters are mixing and reveals in western part of a competitive area. Euryhaline copepods, mainly, adults, take a leading position in plankton in October.
A trend of growth of specific diversity of zooplankton occurs in the Middle and Southern Caspian. It has been supplemented with a new species Acartia clausi for Caspian, which becomes a dominant in certain seasons. An increase of species composition of zooplankton in the 90s has taken place at the expense of undefined species from subgenera Harpacticoida and Cyclopoida, and also freshwater forms.
It seems that an increase of specific diversity of zooplankton also at western coast of the Middle and Southern Caspian, along with favorable hydro-meteorological conditions, is also linked to a decrease of anthropogenic pollution and an increase of sea levels.
4 main zoogeographic groups of animals are distinguished in this fauna of the Caspian Sea - freshwater, arctic, Mediterranean-Atlantic and autochthonous. This fauna is mostly eurythermal and euryhaline. Representative features of the fauna of the Caspian Sea include:
The most typical feature of the benthic fauna is the predominance of autochthonous Caspian species, which often are endemic for the Caspian and are grouped into endemic genera or sub-genera. Caspian endemics and remnants of the fauna of tertiary seas, which existed about 5-6m years ago, refer to them: of molluscs - Dreissena; Micromelaniidae, species Pyrgula; polychete (Polychaeta) (except for newcomers); a part of oligochaeta (Oligochaeta), leeches (Hirudinea); decapods (Decapoda), except for shrimps (Palaemonidae) and crabs (Rhithropanopeus harrisii); turbellarians (Turbellaria) (some species); Cumacea; a large part opossum shrimps (Mysidacea) and scuds (Amphipoda); sponges (Demospongiae); pearlweed (Bryozoa) etc. (Dumont, 2000).
The second group consists of generative-freshwater species, which invaded the Caspian during its desalinization and adapted to live in brackish water. This fauna is rather poor and is represented by separate species of larvae of chironomids (Chironomidae), oligochaetas (Oligochaeta), freshwater molluscs - Dreissena, and among microfauna – of some species of turbellarians (Turbellaria), ostracods (Ostracoda) and benthic rotifers (Rotatoria) (Kasymov, 1987, 1994).
The third group consists of arctic species, which invaded the Caspian from northern seas in later glacial period, about 10-12 thousand years ago and nowadays are widespread in northern seas. These are some species of polychetes (Polychaeta), part of opossum shrimps (Mysidacea) and scuds (Amphipoda).
The fourth group is made of Mediterranean species, which invaded the Caspian from Azov-Black Sea basin independently or were introduced by people. These include: molluscs - Mytilaster, Cerastoderma, Abra, warms - Nereis, crustaceans - shrimp (Palaemonidae), crab (Rhithropanopeus harrisii), balanus (Balanus), 2 species of pearlweeds (Bryozoa), etc. (Agamaliyev, 1983; Kasymov, 1987, 1994).
The main part of benthic organisms lives on or in the seafloor (epi- and endobenthos). These are usual representatives of periphyton (fouling) - sponges (Demospongiae), pearlweed (Bryozoa), worms (Vermes), barnacles (Cirripedia), bivalves (Bivalvia), Mytilaster, Dreissena, infusorias (Infusoria), and also nektobenthos (shrimps - Palaemonidae, opossum shrimps - Mysidacea) and planktobenthos (copepods - Copepoda and cladocerans - Cladocera, rotifers - Rotatoria).
Factors defining geographical distribution of benthic animals include:
In regards to salinity, 4 ecological groups are distinguished in the benthos of the Caspian Sea:
An increase of Caspian levels and desalinization of its waters have made changes into a qualitative composition and quantitative parameters of hydrobionts. In all regions of the sea, the significance of organisms of freshwater and brackish water complexes has increased. Replacement of salt-loving organisms happened in benthos of the Northern Caspian, and in the Middle and Southern Caspian, the greatest development of benthic invertebrates was observed during the spell of an increased discharge of the Volga.
Of the total number of species marked in foulings of the Caspian Seas, only 8-10 play an essential role in shaping fouling. These are Balanus improvisus, B. Eburneus, Mytilaster lineatus, Cordylophora caspia, Perigonimus megas, Conopeum seurati and Rhithropanopeus harrisii tridentatus. Purely Caspian fouling fauna is qualitatively poor and amounts only to five animals species - fouler (Dreissena polymorpha, D. Elata, Cordylophora caspia, Corophium curvispinum, C. Robustum). An essential part of foulers are exotic species.
Endemic fauna was the richest and most diverse (Dumont, 2000). It was represented by: 4 species of Spongia, 2 species of Coelenterata, 29 species of Turbellaria, 3 species of Nematodes, 2 species of Rotatoria, 2 species of Oligochaeta, 4 species of Polychaeta, 19 species of Cladocera, 3 species of Ostracoda, 23 species of Copepoda, 20 species of Mysidacea, 1 species of Isopoda, 68 species of Amphipoda, 19 species of Cumacea, 1 species of Decapoda, 2 species of Hydracarina, 53 species of Mollusca, 54 species of fishes, and 1 species of mammals.
The large number of species are of freshwater origins. Such species constituted the biggest part of such groups as Rotatoria, Cladocera, Copepoda and Insecta. As it was mentioned above, fishes and crustaceans possess the greatest number of species in the Caspian Sea.
In Derzhavinó’s (1951) and Zenkevichó’s (1963) opinion, ichthyofauna used to consist of 78 species. The family Petromyzonidae had 1 species, Acipenseridae 5, Clupeidae 9, Salmonidae 2, Esocidae l, Cyprinidae 15, Cobitidae 2, Siluridae l, Gadida 1, Gasterosteidae l, Syagnathidae1, Atheriiudae 1, Percidae 4, Gobiidae 30, Mugilidae 2, Pleuronectidae 1, Poeciliidae 1 species. According to fresher data of Y. N. Kazancheyev (1981), the total ichthyofauna of the Caspian Sea amounts to 123 species (76 species and 47 subspecies), referring to 17 families and 53 genera. In comparison with other southern seas (Azov, Black, Mediterranean), the ichthyofauna of the Caspian Sea is extremely poor and consists of representatives of autochthonous (63 species and subspecies), Mediterranean (5), arctic (2) and freshwater (56) complexes. Some species have populated the Caspian as a result of human activities.
The distinctive feature of the Caspian ichthyofauna is its high endemism, observed from the category of a genus up to the level of a subspecies. Early separation of the Caspian Sea from the World Ocean has ensured a high level of endemism of its ichthyofauna. According to Kazancheyev (1981), the number of endemics at the level of a genus make 8.2 %, species - 43.6 %, subspecies - 100%. The greatest number of endemic of forms belongs to the families of herrings and gobies, though they occur also in other systematic groups. In general, the Caspian is inhabited by 4 endemic genera, 31 endemic species and 45 endemic subspecies. This has allowed L.S. Berg to single out the Caspian into a special ichthyo-geographic subregion (Kazancheyev, 1981). The active speciation processes in the Caspian Sea are largely related to special hydrological conditions in geological past and present. Repeated transgressions of the sea, its salinization and desalinization promoted formation of new species and subspecies and as well as various biological and ecological forms and races.
One of the endemic species of fauna is the Caspian Seal (Phoca caspica Gmelin), the smallest existing varieties of seals and the nearest relative of northern earless seals ð. Puso, endemic and the only mammal in the fauna of the Caspian Sea. This seal ranges almost in all parts of the sea, periodically visiting deltas of the rivers Volga and Ural (Badamshin, 1966, 1969). Considerable concentrations of seals occur on shell islands in eastern part of the Northern Caspian and on sandy spits of the Southern Caspian in autumnal period (October - November) (Krylov, 1982, 1986). However, the greatest concentration of seals is observed on the ice cover in the Northern Caspian during reproduction and molting in wintertime (Mammals of Kazakhstan, 1981). In 1993, the Caspian seal, according to the classification of the IUNP (International Union of Nature Protection), was referred to vulnerable species and listed in the Red List of the IUNP. The magnitude of the population of the Caspian seal has dwindled from approximately 1.5m animals in the beginning of the 20th century up to 360-400 thousand animals the end of the 80s (Krylov, 1989; Krylov, 1990).
In compliance with Derzhavin (1951) and Zenkevich (1963), the crustaceans used to include 114 autochthonous species:
The biodiversity of molluscs is also great. From 57 up to 70 autochthonous species are registered in the Caspian Sea.
Gastropoda included several species from 4 families: Neritidae - 2 species Theodoxus, Hydrobiidae - 3 (?) species, Pyrgulidae - 26 species, Planorbidae - 1 species, which is called Planorbis eichwaldi and this is the only representative of endemic Pulmonata in deep water parts of the Caspian, which somehow managed to survive deep-water oxygen depletion caused by ancient transgressions.
Bivalvia also includes several species from 2 families:
According to data published by À. Chesunov in 1978, the number of species of free living Metazoa in the Caspian Sea is more than 542 and not 476.
4 species of Porifera are known in the Caspian: 2 species of the genus Metschnikovia, and also Protoschmidtia flava and Amorphina caspia. Coelenterates Polypodium hydriforme is the parasite of sturgeons caviar. The jellyfish Moerisia pallasi is a Caspian endemic.
A hydroid without a medusoid stage - Cordylophora caspia ivaded the Baltic Sea via channels last century and then it spread to other places. Nowadays, this species is a cosmopolite. At present, Cordylophora caspia dwells almost everywhere. For example, this species originating from the Caspian Sea now lives in coastal waters of Northern and Southern America, China, Australia and New Zealand. Probably, this species was the first invader from the Caspian.
3 species of Polychaeta from the family Ampharetidae are known in the Caspian Sea. One species - Hypaniola graizi was found in the vicinity of Woods Hall in coastal waters of the USA. This species is another example of invaders from the Caspian.
There are many invaders from the Caspian Sea into the Volga. According to Birstein, 44 species of invertebrates are found: 1 species of Isopoda, 26 species of Amphipoda, 10 species of Cumacea, 6 species of Mysidacea, and 1 species of Decapoda. 18 fish species have the Volga from Caspian. The most well known invaders from the Caspian into freshwaters are Cordylophora caspia, Polypodium hydriforme, Dreissena polymorpha, Hypania invalida, H. Kovalewskyi, species from genera Theodoxus and Melanopsis. At present, not only Cordylophora caspia, but also Victorella pavida becomes a cosmopolitan species. Today, both organisms range in coastal waters of Northern and Southern America, China, Australia and New Zealand.
Nowadays, there are a lot of Caspian species invading the Baltic Sea: a fish - goby, some Cladocera from the genera Cercopagis and some Mysidae. From the Baltic Sea, Mysidae travels to fresh waters and potable water reservoirs in Europe, and Cercopagis has reached Lake Ontario.
Dreissena polymorpha also has invaded Europe and America from the Caspian Sea, but it occurred in the beginning of the 19th century. The channel Dniepr-Bug was opened in 1803 and in 1824, Dreissena was found in England, then in 1826 in Holland, in 1833 in France, in 1835 in Germany and in 1892 in Spain and Portugal. Recently, in the 20th century, Dreissena entered into America, creating many problems.
The real biodiversity of the Caspian Sea both of plants and of animals is not known today. There are still many new species and subspecies, waiting of description.
Many present marine animals like Scyphozoa, Anthozoa, Ctenophora, Gordiacea, Gastrotricha, Kinorhyncha, Sipunculida, Phoronidea, Loricata, Scaphopoda, Tanaidacea, Pantopoda, Tardigrada, Asteroidea, Ophiuroidea, Echinoidea, Holothurioidea, Chaetognatha, Ascidiacea, Appendicularia and Acrania are not known in the Caspian. But some of these organisms, which are capable of osmoregulation and which are the most euryhaline, can invade into the Caspian. There are a lot of freshwater and brackish water species ranging in estuaries of Caspian tributaries. If we only include names of all these organisms into a list of species and in addition to if we find new unknown endemic species, then the biodiversity of the Caspian could be greater than that of Baikal! Zenkevich included approximately 450 species in his list of free living Metazoa in 1963, Chesunov included approximately 550 species in 1978, and Kasymov included approximately 950 species in 1987. It is possible to imagine that the real number of free living Metazoa is approximately 1500 or even 2000 species. Speciation in the Caspian Sea has created a general high level endemism (approximately 42-46 %) which is a little bit lower, than in Baikal (approximately 54 %). The smaller number of endemics in the Caspian is, probably, related to loss of the Caspian deep-water fauna. But it is possible, that after conducting special researches, the number of endemic species in the Caspian can increase and approach that of Baikal! In our opinion, deep-water oxygen depletions during transgressions of the ancient Caspian sharply reduced the number of Caspian endemics. Only in the Caspian, some groups of animals, which were subject to significant adaptive radiation, had a level endemism close to 100%. If Caspian deep-water endemics had survived, then today the biodiversity of the Caspian would be the greatest in the world for continental water bodies.
What should we, first of all, do to preserve the biodiversity of the Caspian Sea?
The bioresources of the Caspian Sea are of high economic value. Many species of fishes, crawfishes, shrimps, seal certain waterfowls, some wild animals, living in the coastal zone are used for commercial purposes. Each region has own species composition of used animals reflecting local peculiarities.
Fisheries are an important activity in coastal regions of all Caspian littoral countries (Ivanov, 2000; Ivanov, Socoloskiy, 2000). In the recent past, some 500-600 thousands tons of fishes used to be annually landed, and the main part of yields used to constitute such valuable fish species, as Beluga, Russian sturgeon, stellate sturgeon, sterlet, Caspian inconnu, anadromous and marine herring, zander, Caspian bream, carp, Caspian roach, catfish, asp common, kutum, etc. Such a position remained to the beginning of the 50s of the present century, when hydroconstruction, yearly redistribution of river runoffs, restriction of vernal discharges, abstraction a great amount of water for irrigation and other economic needs, exploitation of watersheds without effective means of fish protection, and water pollution have led to deterioration of conditions of reproduction of valuable fish species of the Caspian basin, reduction of their stocks and catches. For example, if in 1932-1936, yearly catches of commercial fishes (without sprats) of all fisheries (except for Iran) made 394 thousand tons, in 1951-1955, they decreased up to 283 thousand tons and in 1990-1995 - up to 81 thousand tons.
Since 1950, in order to compensate unharvested valuable commercial fishes, sprat fisheries in the Middle and Southern Caspian has intensified. In the 1960-1980s, yearly 300-400 thousand tons of sprats were landed. A substitution catches of valuable fish species by the sprat. Catches of sprats by the Caspian littoral states (except for Iran) in 1997 constituted about 90 thousand tons.
Transboundary commercial fish species of the Caspian Sea include sturgeons (5 species), sprats (3 species), shads and mullets (2 species). Gobies and sandsmelts, which serve as food for predatory fishes, live everywhere.
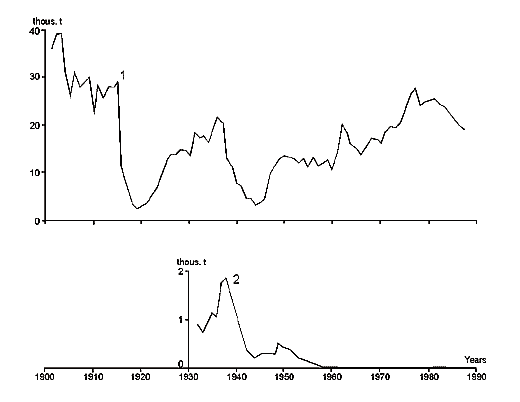
Fig. 23. Dynamics of sturgeon catches (1) and salmons (2 in the Caspian Sea, thousands of tonnes. (Gurevitch, Lopatin, 1962; Catches of Fish …, 1985, 1986; Kaspiiskoye Morye. Ichthyofauna …, 1989).
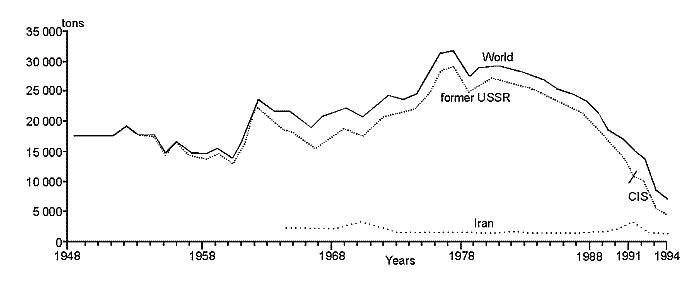
Fig. 24. The world-wide catch of sturgeon fishes, 1948–1994.
Sturgeons are of the greatest consumer value (Fig. 23, 24). Sturgeon meat, derivatives and caviar have great demand international markets. As a result, sturgeons became an attractive target for poachers. Main harvested species of sturgeons are the Russian and Persian sturgeon, Beluga and Stellate sturgeon. The ship sturgeon is rare. It’s fished only in Kazakhstan. The spawning grounds of the ship sturgeon in the Kura have lost its commercial importance. In Azerbaijan and Turkmenistan, the ship sturgeon is listed in the Red Book. The sterlet is a freshwater species. It ranges only in the Volga.
Three sprat species inhabit the Caspian Sea: common, big-eyed and anchovy. Habitats of the two latter species are the Middle and Southern Caspian, the common sprat occurs all over the Caspian. The anchovy sprat is the main fishing target (85 % of the total catches). Sprats are mainly harvested on the shelf of the Southern Caspian and in smaller volumes in the Middle Caspian.
The commercial stocks of herrings, which used to be considerable in the 60s, have sharply dropped in the second half of the 20th century. Only recently, they have become to restore. Now, black-backed shads, Dolginka shad, Caspian and big-eyed shads, and Southern Caspian herrings are commercial species of herrings. Herrings are harvested in the delta of the Volga (black-backed shad), at Azerbaijan, Turkmen and Iranian coasts. Lack of specific fishing devices is a restriction to herring harvesting in open sea, because young sturgeons are by caught. Mullets (golden and gray) were introduced into the Caspian Sea from the Black Sea some 30 years ago. Due to introduction of these species, a large herd of valuable commercial fishes was established. Its main inhabitants include the Southern Caspian. The maximum catches of mullets in Turkmenistan waters were recorded in the middle of the 50s (up to 550 tons). In compliance with experts’ conclusions, its stocks are rather great (catches can be increased up to 800 tons), but they are developed extremely badly. Recently, yields have hardly exceeded 40-50 tons. Iran and Azerbaijan are also catching mullets.
From other anadromous fish species, salmon (2 species), asp common, kutum, barbel and zahrte are fished in the Caspian Sea. There are local shools of these species. They stick to certain habitats.
Salmons are represented by two species: Caspian trout (subspecies of the Caspian trout, dwelling in the basins of the Baltic and White Seas) and Caspian inconnu. The feeding grounds of the salmon are located along western and southern shores of the Middle and Southern Caspian at the depths of no more than 40-50 m. The Caspian trout forms several shoals, which are linked to basins of several rivers feeding the Caspian: in the Southern Caspian it is the Kura, Lenkoranka and Astarinka. Prior to damming of the river Kura, it was the main Caspian trout fishery in the Caspian (0.5-3.5 thousand fishes). In order to compensate damage inflicted to salmon stocks by damming of the river Kura, two hatcheries were constructed in Azerbaijan: Chaykend (in 1954) and Chuhur-Gabala (1956). These hatcheries annually release 0.6m salmon smolts. Now, the Chaykend hatchery is not functioning, and the efficiency of the Chuhur-Gabala hatchery has considerably lowered to several scores of thousands of fries per year, because of a considerable lack of brood stocks. A trend of disappearance of this species, in view of uncontrollable poaching, is observed. Now, the Caspian trout is listed in the Red Books of Kazakhstan, Turkmenistan and Russia.
The population of the Caspian inconnu, which is used to a commercial species, has reduced to a minimum. Now, it is fished only on the Volga. In the same time, a disastrous reduction of its population is observed. The total catch of 1998 constituted 10.2 tons. At present, the Caspian inconnu is fished only for monitoring and for reproduction purposes. The Caspian inconnu is listed in the Red Books of majority of Caspian littoral states.
Kutum, barbel and zahrte are inhabitants of the Southern Caspian. They form local shoals preferring either western or southern coasts. In the Northern Caspian, the kutum almost does not occur. Therefore in Kazakhstan and Russia, it is listed in the Red Books.
The Asp common is a widespread predatory species harvested in all Caspian littoral states.
Semi-anadromous and freshwater fishes of the Caspian basin - Caspian roach, Caspian bream, zander, common carp, catfish, pike, crucian carp, rudd, tench, silver bream, and perch – are traditional and important fishing objects. The life cycle of typical semi-anadromous fishes (Caspian roach, Caspian bream and zander) is linked to lower stretches of the rivers Volga, Ural, Terek, Kura, Atrek and other rivers, where they reproduce, and estuarine areas of the sea - feeding grounds of young and adult fishes. The common carp and catfish should refer to semi-anadromous fishes, as they migrate and a part of their populations, apart from an avant-delta, fattens in the sea. Invaders such as the silver carp and the white grass carp are fished in southern regions of the sea.
Freshwater species are fished in river estuaries: a pike, rudd, tench, crucian, catfish, loach, perch etc.
In the 1990s, as a result of uncontrollable poaching and decrease of artificial reproduction, the population of all sturgeon species (Beluga, thorn, sturgeon, Stellate sturgeon), Caspian trout, Caspian inconnu, khramulya, Danubian bleak, barbel and zahrte has decreased. These species, especially sturgeons and salmons, are threatened in connection with a considerable decrease of fries releases from hatcheries.
The water regime of the river Atrek is a leading factor for the reproduction of semi-anadromous fishes (Caspian roach and carp) in southeastern part of the Caspian. The regime of the river is extremely unstable. For example, in 1984, 1986, 1990 and 1991 water didn’t reach lower reaches of the river, and spawning didn’t occur.
Two crawfish species are harvested in Turkmenistan – pachypods and palpods. In recent years, it was at a very low level, though their stocks are estimated to be quite high, and up to 50 tons can be yielded per year. Now, they are harvested in the region of Kara-Bogaz-Gol, settlement Karshy, however, catches do not exceed 3-5 tons.
Under obvious reduction of fish catches in the Caspian Sea, they do not correspond with available stocks and are accounted for an economic recession in the fish industry. There are no real threats for majority of commercial species.
Nevertheless, fishing, along with other factors (damming of rivers, pollution), has led to disappearance of some fish species from catches. In the 20-40s, Caspian lamprey, Volga herring, Caspian trout, Caspian inconnu, which total catches in the Caspian basin amounted to 80 thousand tons, used to be usual commercial species. Now, these fishes are listed in the Red books of the Republic of Kazakhstan, Russian Federation and other Caspian littoral states.
The seal is the only mammal inhabiting the Caspian Sea. It has a long commercial history characterized by yield fluctuations during 2-3 centuries. Only in the 20th century, the range of fluctuations of the level of seal harvests reached several hundreds thousands animals. Further intensification of production could have led the loss of the valuable commercial species. Only, strict control and change of the profile of the industry to fur production, conducted in the 1966-1970s, have allowed for stabilizing the magnitude of the population of the Caspian Seal at the level of 500-600 thousand animals with the brood stock in the amount of 90-100 thousand females.
Specially conducted researches showed that within the last decades, certain destabilizing processes have been observed in the population of the Caspian Seal (Ivanov, Socoloskiy, 2000). The mass examination of animals before a breeding season at autumnal rookeries in 1989-1990 has revealed that up to 73.4 % of females dropped out of reproduction for different reasons (pure barrenness, pathology etc.), collectively called "barrenness". A similar situation repeated in five years. Naturally, such sharp changes of barrenness could help influencing on the magnitude of population, which since 1986 to 1995 reduced by approximately 20%. The crisis of reproduction of the population, in turn, is a corollary of unfavorable processes happening in the ecosystem of the Caspian Seas. This resulted in mass deaths of seals in 2000, which begun in the northeast of the Caspian and spread to all over the sea. A prospective reason of the deaths is distemper on the background of cumulative polytoxicosis and salmonellosis.
The seals are bagged on ice in wintertime in correspondence with allocated quotas. Largely, newborn pups (white-coat seals) with fluffy white fur are hunted. In connection with economic difficulties, seal hunting has been stopped by all countries since 1997, except for Russia. Russia has stopped hunting since 1998.
A part of commercial species of mammals is closely connected to in its distribution to coastal belt of bushes and reedbeds. Brown hare, wild rabbit, coypu, forest dormouse, wolf, golden jackal, fox, American raccoon, least weasel, beach marten, Eurasian badger, otter, reed cat, steppe cat, lynx, goitred gazelle and other refer to such species. These mammals are of high importance for human economy and as food items. The majority of valuable mammals are predators.
Of wild hoofed animals of the Caspian coast, the wild boar is on the first place by its population and is an important and interesting game animal.
The largest predator at the coast of the Caspian Sea is the wolf. It occurs in all zones. Its habitats are very diverse, as it is easily adapting to all landscape zones. The distribution of wolfs within their extensive habitat depends on availability of wild and domestic hoofed animals. The value of wolf’s fur is rather low.
Golden jackal is the most widespread species. There are a lot of golden jackals in Lenkoran and Samur-Devichy coastal area of the Caspian Sea. They are mainly found on lowlands, in dense reedbeds and bushlands on shores of rivers and lakes. They often occur around human settlements. Although, the golden jackal is of low value as a fur animal, nevertheless, its fell has quite good qualities. It is warm, durable and light, though a little coarse and, may be, not so beautiful. The fur coats made of golden jackal fell are highly appreciated by Kazakhs. Such fur coats are incomparably warmer and lighter, than sheepskin. Fells of this predator are used in the light industry for production of caps, collars and fur coats.
The fox belongs to the main objects of the fur trade. In general, the fox belongs to a number of useful wild animals, as, except for valuable fur, it brings a great benefit by killing rodents. On the contrary, the harm caused by destruction of useful birds and animals is insignificant (Novikov, 1965;.Hidayatov, 1967).
Of fur animals, the otter, lynx, steppe and cane cat, beach marten and least weasel are hunted in the Caspian littoral states. The otter has the most valuable fur. The fur cats is of low value and is produced only incidentally in small amounts.
The lynx has a quite valuable fell, but is produced in small amount and is of low value in the fur trade.
The least weasel ranges almost in all over the Caspian coast. Its fell is of low commercial value. It is hunted incidentally. The least weasel is of high benefit since it hunts mice.
The coypu is a valuable commercial animal of the rodent order. The coypu has valuable meat and even a more valuable fur distinguished with a dense underfur. A dense hair is hard to get wet, that gives an animal a possibility to stay longer in water.
The corsac and steppe polecat is hunted for fur in Kazakhstan.
Three herds of saiga (Saiga tatarica) live in northern cis-Caspian: Usturt (between the Caspian and the Aral), Guryev (interfluve of the Volga and Ural) and Kalmyk herd (Kalmykia). After imposition of the ban for hunting saigas in Russia (1991), their population has reached 300 thousand animals. A lowered sheep breeding positively influenced on the increase of saiga population. In Kazakhstan, the saiga is an important commercial species. There are Usturt and Volga-Ural populations of saigas in Kazakhstan. The magnitude of populations is maintained at the level of 250-275 thousand animals during the last decade.
Game shooting is widespread in all Caspian littoral states. Local population uses their meat and down.
At Azerbaijan coast, during migration and wintering, the main mass of birds, which are allowed to hunt, consists of 14 species: gray goose, mallard, teal, gray duck, vigeon, pintail-shoveler, red-crested pochard and pochard, tufted duck and scaup, goldeneye, large merganser and coot.
In Turkmenistan, the main hunting areas are located at the central part of the coast in the Krasnovodskiy gulf. Bird hunting at northern and southern reaches of Turkmen coast (hunting and poaching) during recent years has increased. The cost of seasonal (almost for 7 months of migrations and wintering) hunting of 1997-1998 in market prices for that time at both sites is evaluated in the amount of about 500 thousand dollars. In regards to species, this amount of hunted birds is subdivided as follows: coot - 59 %, diving duck - 19 %, river ducks - 15 %, other species (grebes, large cormorant, seagulls and waders) - about 7%. The indicated figures of hunting make an insignificant portion of all migrating and wintering mass of Western Siberian-Caspian-Nile populations of waterfowls and shore birds. However, increasing extent of such hunting is worrying: 1974-1990 in average 1.2 %, 1991-1996 - up to 3.5 %, in a season of 1997-1998 - up to 4.7 %, i.e. for the last 8 years it has grown by a factor of 3.5.
Ducks constitute the majority of game birds also in other Caspian littoral states.
Due to uniqueness and diversity of natural environments, many rare species of fauna and flora survived in the Caspian Sea.
In connection with the sea level rise in 1994-1996, habitats of rare water plants have sharply diminished owing to a disastrous change of habitats and lack of seed material in newly formed coastal lagoons and reservoirs.
The biggest part rare and endemic plant species of Russia grow on intrazonal communities of the delta of the Volga and riparian forests of the delta of the river Samur, and also to a unique biotope - sand-dunes of Sarykum, representing a flora refugium of loose sands of ancient Central Asian deserts. In the first case, the main limiting factors for successful growth of species is changes of a hydrological regime of deltas, contamination of reservoirs, and various hydro-ameliorative activities. Water level fluctuations are an indirect reason. This is a negative factor for water plants of the delta of the Volga: Aldrovanda veiculosa, Nelumbo caspica. 11 species occur on small area in the delta of the river Samur, where unique liana forests of a tertiary age survived from the tertiary times. The problem of protection of these species is closely related to the problem of preservation of the biodiversity of forests in the delta of the Samur. Intensified of economic development of coastal and the Samur-Devichy lowlands and increased recreational load result in degradation of a unique intrazonal system and disturbance of its formed functional connections. 20 rare species of plants are characteristic for zonal dry steppe and desert communities of Kalmykia and low-lying areas of Dagestan. These species are widespread on marine terraces of various levels (the maximum number of species refers to New Caspian terrace). The main limiting factor is the anthropogenic load on the territory, which has recently increased. Unlimited grazing, economic construction, tillage, meliorative works - are the reason of vanishing of zonal communities of steppes and deserts and their replacement by secondary, impoverished species and poorly productive communities. Rare and endemic species become uncompetitive in such situations, as they have low viability.
Population of such species as Aldrovanda vesiculosa, Nelumbo caspica, Diandrochloa diarrhena, Marsilea aegyptiaca, Trapa natans is directly related to water level fluctuations. All of them grow in the delta of the Volga, at the territory of the Astrakhan reserve.
Experts offer to list in the Red Book additional 16 species in Azerbaijan and 8 species in Kazakhstan. The species requiring protection in the Caspian region include chosen from critically endangered species - Rubia cretacea, from endangered - Aldrovanda vesiculosa, from vulnerable - Linaria cretacea (National strategy …, 1999). Aldrovanda is a water plant growing in the river Ural, Rubia and Linaria – at chalk outcrops on riverbanks of the Emba and at Usturt. All these species used to be rare in the 60s. The single discoveries were partially made in 1990-1996, but the range of the species is not known, additional researches are needed. Besides, Convolvulus persicus, Stipa pseudocapillata, Artemisia gurganica, Linaria leptoceras are offered for protection (Safronova, 1996).
Rare and vanishing species - special categories of insects of the regional fauna. Twenty rare species from the Red Book of Kazakhstan (1991) inhabit in northern cis-Caspian. But this list does not include all rare and vanishing insect species of this region. Only of lepidopterous, there are about 100 rare species, which makes almost 20 % of the total species composition (Smiths, Martynova, 1954). This circumstance should be considered during organizations of environmental measures and preservation of biodiversity i.e. not to limit attention only to species listed in the Red Book, but to take into account the whole taxonomic composition of the regional entomofauna.
Data on entomology of other coastal regions are not available.
Of 20 species of these animals at the Iranian coast, 17 species or 85% are listed as threatened species. Use of these animals as the source of proteins is considered to be the main reason for the decrease of their population, particularly, this refers to an edible species - Rana.
Human activities have negatively affected habitats of amphibians. Land development and drainage wetlands pose a huge problem for amphibians. Pesticides and herbicides also negatively influence on habitats of amphibians. Some of these organisms are highly sensitive to pollution. They are used as indicators for guarantee safety of water. An amphibian will have health problems, if it eats polluted insects. Pollutants seeping in water ecosystems not only cause an environmental problem, but also jeopardize the habitats of tadpoles.
In other Caspian littoral state, the diversity of amphibians is rather low, and accordingly the number of rare species is insignificant.
The Red books of Russia and Kazakhstan includes 8 (21 %) species reptiles inhabiting in this region. Another 4 species are listed in II and III Appendices to the Bern Convention. The majority of rare species (7) inhabit at the territory of Dagestan. The most rare taxon, which is at the brink of extinction, is eastern Caucasian subspecies of the Mediterranean turtle (Testudo graeca ibera) and the western sand boa (Eryx jaculus). The Mediterranean turtle inhabit in dry steppes in semi-deserts at the territory of Dagestan, in the same time, it also penetrates into mountains up to the height of 1100 m. The western sand boa is found only in the south of Dagestan in sheep’s fescue-wormwood steppes. As well as the previous species, it spreads into mountains up to the height of 1500-1700 m. The large whip snake (Coluber caspius) inhabits stony and clayey scarps of rivers and ravines in steppes and deserts, and shore of lakes and rivers. In Kazakhstan, it occurs only in the Volga-Ural interfluve. The four-lined snake (Elaphe quatuerlineata) is rare species with diminishing population, which is an inhabitant of various landscapes (dense fixed and half-fixed sands, clay and stony deserts). It sometimes settles in human constructions. This species is captured for keeping in captivity and needs protection.
Mammals inhabiting the coast zone of the Caspian Sea are rather sparse. Of them, more than 35 species are listed in national Red Books as rare and vanishing species, requiring special protection. These are the Bobrynsky’s bat, marbled polecat, goitred gazelle, Usturt mountain ram and Asiatic wild ass in the Republic of Kazakhstan, the lesser white-toothed shrew, lesser horseshoe bat, porcupine, marbled polecat, otter, steppe cat, lynx, seal and goitred gazelle in Azerbaijan, goitred gazelle and otter in Turkmenistan, and 18 species in Russia, of which the most endangered are the desman, European mink and Caucasian otter. Of 19 species of mammals at the coast of Iran, 8 species or 42 % are listed as threatened. The wolf, hyena, Caspian seal are rare species at the Iranian coast. These animals face shrinking habitats or they may live in a special ecosystems, which nowadays loses its features. The main reason of this problem could be destruction of a green habitat, deforestation or disturbances in rivers. All the above mentioned problems have contributed to destruction of the trophic pyramid or have extremely reduced it.
There are different reasons of scarcity of rare species. For example, the population of marbled polecats depends on major food organisms - ground squirrel and sanderling. The hunting and mass grazing of livestock have affected the population of the goitred gazelle, which used to be a commercial species in past. Before the 30s, up to 200 thousand animals of these species used to range in Kazakhstan, nowadays - up to 50 thousand animals, half of which range in the Mangistau district. At present, the population of the goitred gazelle at the peninsula Buzachy constitutes 16-20 thousand animals (Red book of Kazakhstan, 1996). The single encounters were registered in lows between sand dunes at the peninsula Dardja in Turkmenistan and in Azerbaijan, where it also is listed in the Red Book.
The population of desmans inhabiting the delta of the Volga is adversely affected by water level fluctuations and intense use of floodplains by local population and fixation of nets during fishing. Hunt for the desman was banned in 1920.
The European mink (Mustela lutreola) is a very rare and vanishing animal. It is widespread in the delta of the Volga. The population is negatively influenced, apart from anthropogenic factors, by the competition with an ecologically close introduced species – the American mink (Mustela vison).
The otter is listed in the Red Books of Turkmenistan and Azerbaijan. At the same time, in the Astrakhan district, including in the delta of the Volga, the hunt for the otter is permitted in correspondence with allocated quotas. The Caucasian otter - a rare subspecies of the widespread species inhabits the river systems of the Terek and Sulak in Russia (Dagestan).
The Turkmen Asiatic wild ass inhabits eastern coast of the Caspian. It is a vanishing animal of the global fauna of the family of horses. In Kazakhstan, it was exterminated in the 30s, then it was introduced on island Barsakelmes in the Aral sea in 1953. In 1991, it was introduced in the Actau-Buzach sanctuary at the peninsula Tub-Karagan.
Of birds inhabiting the coastal of Azerbaijan, 41 species are listed in Red Books. Of these 29 species are listed in the Red Book of Azerbaijan (including 9 species in the Red Book of IUNP), 12 species are listed in the Red Book of IUNP.
31 species of birds listed in the Red book of the Republic of Kazakhstan are found at the Caspian coast and in adjacent inland areas. Majority of them are inhabitants of water and coastal ecosystems (pink and curly pelican, yellow, small and cattle egret, spoonbill, glossy ibis, flamingo, whooper swan and small swan, red-breasted goose, marbled teal, oxyura, Asiatic white crane, purple gallinule, great black-headed gull, fish hawk and white-tailed sea eagle). Diversity of rare birds of desert landscapes is a little bit poorer - 13 species (sociable plover, sandgrouse, pallas sandgrouse, crane- oxyura, great bustard, little bustard, houbara bustard, short-toed eagle, imperial eagle, golden eagle, steppe eagle, saker falcon and eagle owl).
At the same time, the main population of rare birds of Kazakhstan are linked to the Caspian sea and its coastal zone - flamingo (up to 35 thousand), pink pelicans (up to 2 thousand), small white heron (up to 1.5 thousand), glossy ibis (up to 0.6 thousand), and in wintering the greatest number of white-tailed eagles (up to 350 birds) is concentrated here.
The list of rare and vanishing bird species of the Russian coast includes 45 species, which makes about 17.6 % of the ornithofauna, and includes species protected at the territory of Russia and Kazakhstan. Of these, 12 species (27 %) are listed in the Red book of Europe in the category "endangered" (E), "rare" (R) and "vulnerable” (V); 14 species are listed in the Red book of IUNP (categories E, R, V); 28 species are included in Appendix II of the Bern Convention. The ornithofauna of Dagestan and Astrakhan province are the richest with rare species. Of 45 species, 24 (53 %) are closely connected to seashore and use it as a feeding and / or nesting ground.
The population of the curly pelican (Pelecanus crispus) is the most ailing. Its population probably does not exceed 300 birds (the species is listed in the Red book of IUNP, category "E"). Places of concentration: the delta of the Volga (mainly between Kirov and Gandurinskiy channels), valley of the Terek (Kizlar and Agrakhan gulfs), and in Kazakhstan sector in the vicinity of the spit Zhiloy.
The population of the pink pelican (Pelecanus onocrotalus), which exceeds 2 thousand birds, is much higher. Few of them regularly nest at the territory of Russia, on lake Manych-Gudilo and irregularly in the delta of the Volga. In the Kazakhstan sector, where the population of pelicans is much higher, these birds concentrate mainly between the mouth of the Ural and the spit Zhiloy and in estuarine region of the Volga. The Russian coast Caspian is also inhabited with the following especially rare species the small cormorant (Phalacrocorax pygmaeus) (category "K" in the Red book of IUNP), flamingo (Phoenicopterus roseus), cattle egret (Bubalcus ibis), spoonbill (Platalea leucorodia), glossy ibis (Plegadis falcinellus), purple gallinule (Porphyrio porphyrio), Asiatic white crane (Grus leucogeranus) (category "V" of the Red book of IUNP), sociable plover (Chettusia gregaria) (category "R" of the Red book of IUNP), European shikra (Accipiter brevipes), European short-toed eagle (Circaetus gallicus gallicus), pallas’s sea eagle (Haliaeetus leucoryphus), white-tailed sea eagle (Haliaeetus albicilla), and great bustard (Otis tarda).
Of 289 bird species inhabiting wetlands of Turkmenistan, 43 refer to rare and endangered. Of these, 24 species are listed in the Red Book of Turkmenistan, the remaining 19 species need protection:
At least 30 bird species are registered in the province Gilan (Iran) as threatened, they make 50 % of the bird population in this area. 40 % of threatened birds are game birds, Anatidae amount to 23 % of these species.
These species are considered to be highly sensitive, and are listed in the list as rare species. In past, they used to be found among the population of birds in the province, but recently they have become rare, for many reasons, the main of which is unreliability of the established protection of their habitats. It is necessary to precisely define species of Marmoronetta ongustirostris, which can be found in Iran, but disturbance of their habitats in Gilan forces this population to change their migratory route, instead of selecting this area as a sanctuary. The effect of pesticides and other toxic chemicals endangers reproductive functions of birds.
Peculiarities of distribution of species in the Caspian cause different frequency of occurrence of fishes in different regions. Species, which are rare in certain regions of the sea, can abundant in other.
The Caspian lamprey was listed in the Red Books of all littoral states. It was awarded the status of the II category – a species, diminishing within its habitat. It is the only representative of the order lamprey in the Caspian Sea. It used to strewn along the entire coast, from Azerbaijan up to the Northern Caspian. It spawns in rivers running for hundreds kilometers upstream. In past, it used to be a usual fishing object. The population is limited hydroconstructions on rivers, which restrict opportunities for reproduction.
Salmons, which also used to be commercial species, are very rare in the Caspian Sea. They are represented by two species: the Caspian trout and the Caspian inconnu. The Caspian trout is listed in the Red Books of Russia, Kazakhstan, and Turkmenistan. In Azerbaijan and Iran, salmons are characterized as sharply reducing species.
The Caspian trout was awarded the status of the 1 category. It is a threatened species. The Caspian trout is the representative of a polytypic species, which subspecies inhabit the basins of the Baltic, White, Black, Azov and Aral seas. It is mainly spread in southwestern part of the Caspian Sea and its tributaries (mainly, flowing down from the Caucasian mountains). It spawns in rivers. 410-620 tons per year used to be caught in the 40s, and only 5 tons in the 60s. The main mass of salmons is concentrated in southwestern part of the sea. The reason of the decrease of population is the disturbance of natural reproduction resulting from river damming.
The Caspian inconnu is the representative of the family of whitefishes. It was awarded the status of the 4 category. It is listed in the Red book of IUNP. The Caspian inconnu is one of the subspecies of the nelma, which have a narrow habitat. It is distributed only in the Caspian Sea, mainly in its northern and middle parts and in tributaries (Volga, Ural). The Caspian inconnu lives in the sea and spawns in rivers. In summertime, it sticks to deep-water regions of the sea (up to the depth of 50 ì), in autumn and winter it is concentrated in shallow areas of the Northern Caspian.
In the 30s, catches of the Caspian inconnu in the Northern Caspian reached 1.4 thousand tons per year, by the end of the fiftieth catches had decreased up to 0.4 t. The disastrous decrease of stocks is attributed to damming of the Volga, which almost completely stopped natural reproduction. Later, due to artificial reproduction, the stocks were almost restored, but they are still far cry from being commercial. The major factor limiting its population is the destruction of conditions of reproduction as a result of hydroconstruction. Before the main spawning grounds were located in the feeder of the Volga – the river Kama. Now, part of brood stock spawns down the Volgograd hydrosystem, but the efficiency of these spawning grounds is very low.
The Volga herring is listed in the Red Books of Russia, Kazakhstan and Turkmenistan. It was awarded the status of the 2 category as a subspecies with disastrously dwindling population. The Volga herring returns to rivers (Volga, Ural, and Terek) only during spawning. In the 30s, it used to be the basis part herring fisheries in the Caspian Sea (up to 70 thousand tons per year). Now, it is found extremely rarely. The reasons of the decrease of population are destruction of conditions of reproduction and irrational use.
The kutum is listed in the Red books of Kazakhstan and Russia. This representative of the carp family was awarded the status of the 3 category – a very rare species in the Northern Caspian, with sharply reducing population. The kutum is a subspecies with a limited distribution. The main concentrations occur in southern, southwestern and middle parts of the sea. In the south, the kutum is of commercial importance. In the Northern Caspian, it has become rare a long ago (no reported catches in last years). The decrease of population happens as a result of destroyed of conditions of reproduction in connection with a deteriorating hydrological regime.
The white-eyed bream, ziege, marine zander and Kura ship are listed in the Red Book of Azerbaijan. A decrease of population of the marine zander is observed also in other southern Caspian countries (Iran and Turkmenistan). The Marine zander (Luciopera marina) has almost completely disappeared in the south of the Turkmenbashi gulf, its population is also limited in the region of rocky areas of the sea in Kara-Bogaz-Gol. The disappearance of the marine zander from wetlands of the Turkmen shore (as well as in other areas of the Caspian) especially sped up after intensification o development off hydrocarbons.
In the 90s poaching sharply increased. First of all this had an effect on the most valuable fish species such as sturgeons and salmons. As a result their population has sharply decreased and now the issue of preservation of these species is in the agenda. At the Azerbaijan coast commercial stocks of such fishes, as khramulya, Danubian bleak, barbel, zahrte have considerably decreased. These species are threatened. Azerbaijan experts recommend listing these species as well as sturgeons and salmons in the National Red Book.
In Kazakhstan it is offered to list the sterlet, Volga podust, Caspian barbel and Caspian coach in the Red Book.
In Iranian waters, the Caspian trout, Caspian bream and zander, which need protection are under the threat of extinction in connection with overfishing, destruction of habitats and deterioration of spawning grounds.
In accordance with À. Derzhavin (1951), F. Mordukhay-Boltovskiy (1960) and L. Zenkevichó (1963) in the Caspian Sea, the fauna of arctic origins is represented by 1 species of Polychaeta, 1 species of Copepoda, 4 species of Mysidacea, 1 species of Isopoda, 4 species of Amphipoda, 2 species of fishes and 1 species of mammals.
Arctic invaders are one of the most ancient groups of invaders into the Caspian. Most probably, they have penetrated here during the glaciation period. The following organisms can serve as an example of arctic invaders.
One species of Polychaeta from the family Sabellidae - Manayunkia caspia probably penetrated into the Caspian from the Arctic region. There are many other arctic invaders in the Caspian Sea: Limnocalanus grimaldi, Mesidothea entomon glacialis, Pseudalibrotus caspius, P. Platyceras, Pontoporeia affinis microphthalma, Gammaracanthus loricatus caspius, Mysis caspia, M. Microphthalma, M. Macrolepis, M. Amblyops, Stenodus leucichthys, Salmo trutta, and Phoca caspia. How and when all these organisms invaded the Caspian Sea is not known. There are some hypotheses.
The first – offered by Ekman and Sars (Ekman, 1916; Sars, 1927), contents that it was a result of a direct contact between the Caspian Sea and the Arctic Ocean. At present, this hypothesis is forgotten because of lack of proofs. The same idea was put forward by Gumbalt (1912). He stated that there was a strait between the Caspian and the Arctic Ocean. In scientific literature, this strait was named "Strait of Gumbalt". The second hypothesis was advanced by Grimm and Kessler. They deemed that arctic immigrants arrived from the north to the Caspian with streams of fresh water. Today, this hypothesis is supported by some data, which is sufficient. The third was offered by Guryanova and Pirojnikov. These scientists assumed that immigrants originated in the Kara Sea. There are no direct confirmations of this idea. The last hypothesis was proposed by Berg. It believed that there was a "fish lake" (Berg, 1928). This hypothetical lake stretched from the Baltic Sea up to Ladoga Lake. From Ladoga up to Onega Lake. From Onega up to White lake. From White lake up to the river Sheksna. This hypothetical lake ensured connection of the Caspian Sea with Arctic and Baltic waters. Today, there are a lot of data supporting this hypothesis, but modern scientists have given another title to this hypothetical lake. They name it "Mologo-Sheksninskoye" lake (Zenkevich, 1963). The modern researchers suppose that so-called arctic immigrants have penetrated not directly from the Arctic Ocean, not from the Kara Sea, but from the Baltic and White Seas. All invaders from the Arctic region demonstrate little or do not show any indications of speciation. The low level of speciation could be the evidence of recent invasion or only an evidence of genetic conservatism of all arctic invaders. Several researchers - Yablokov, Dyumon and others suppose that the Caspian seal, Phoca caspica, is a predator holding the uppermost place in the Caspian, is similar, if not identical, to the Baltic seal.
Recently, at the bottom of the Caspian Sea, tusks of a walrus were found in the vicinity of the Absheron peninsula. Probably, this inhabitant of the Arctic region used to live in the Caspian Sea (Vereshagin N.Ê., oral message).
Plankton crustaceans from the family Cercopagidae should, probably, refer to northern invaders. According to data îsmîregularoty and molecular-biological researches of representatives of the genus Cercopagis can not be consider as typical Caspian endemics. These crustaceans refer rather to the group arctic or freshwater invaders of the Caspian, than to true autochthonous fauna of the Caspian. In our opinion, ancestors of the modern Cercopagis could penetrate into residual reservoirs of Parathetis in time of the Akchagyl or Absheron transgressions (2.5-1.1m years ago). Thus, the modern Cercopagis and Bythotrephes, obviously, had a common ancestor, which lived on the margins of the Baltic glaciation shield. A part of ancestors, apparently, remained in Palearctic region and gave origin to B. Longimanus, and another part penetrated into the ancient Caspian, when its waters overflowed far in the north in the vicinity of the mouth of the modern Kama. Here, in the Akchagyl or Absheron sea, a fast evolution of the ancestors of the forms Cercopagis (accelerated phenomena of cyclomorphosis, parthenogenetic and hamogenetic cloning, hybridization, etc.) begun here. These specific evolutional processes, obviously, ensured unusually fast (a bit more than 1m years) and extensive adaptive radiation of Cercopagis.
The introduction of freshwater organisms into the Caspian Sea happened several times during its greatest desalinization. The gastropod molluscs are considered the most ancient freshwater elements. To certain degree this was facilitated by the nature of the salinity of the Caspian, essentially differing from oceanic water by its composition. The number of freshwater invaders in the Caspian is so high, and their presence is irregular that we refer readers to the report prepared Y. S. Chuykov (1994).
According to data of Zenkevich (1963) the fauna of Atlantic-Mediterranean origins is represented by: 1 species of Turbellaria, 1 species of Coelenterata, 2 species of Polychaeta, 1 species of Copepoda, 2 species of Cirripedia, 3 species of Decapoda, 3 species of Mollusca, 2 Bryozoa and 2 (6) species of fishes.
Atlantic and Mediterranean invaders penetrated into the Caspian Sea 3 times:
After this, some more species penetrated into the Caspian Sea through the Volgo-Donsk channel. For example, 6 species of marine algae were found by Zevina. 2 species of marine Cladocera: Pleopis polyphemoides and Penilia avirostris were registered by Mordukhay-Boltovskiy and Aladin.
A delibarate introduction of commercial and fodder water organisms exerted essential influence on the biodiversity of the Caspian. So, according to data of Karpevich (1975), in the period with 1930 on 1970, at least 9 species of fishes were introduced into the Caspian: Glos’s flounder - Pleuronectes flesus luscus, topknot Rhombus maeoticus, mullets Mugil auratus and M. Saliens, white grass carp Ctenopharingodon idella, white silver carp Hypophthalmichtys molitrix, motley silver carp Aristichthys nobilis, chum Oncorhyncus keta, hampback Oncorhyncus gorbuscha. Of other introduced fishes - salmon Salmo gairdneri, striped perch Morone sazatilis, Mugil so-iuy - results are not known yet. Of invertebrates, organisms brought from the Azov-Black sea basins were successfully introduced polychaetous worms Nereis diversicolor, bivalves Abra ovata and shrimp Palaemon elegans.
It is possible that some more atlantic-Mediterranean species are currently invading the Caspian Sea, and will be registered by scientists in the nearest future (Dumont, 1995). The comb-jelly Mnemiopsis leidyi has invaded the Caspian recently (Ivanov etc., 2000). This species of comb-jellies has negatively influenced industrial fisheries in the Black sea and first of all on catches of plankton-eating fishes (Comb-jelly …, 2000). Some researchers from Azerbaijan and Turkmenistan have already informed of observations of the comb-jelly in the Caspian, however, these data were not published or properly confirmed: photos, pictures, and caught and fixed specimens. Recently (the autumn of 1999), Russian researchers from KaspNIRKH, Ushivtsev and Kamakin managed at the depth of 30 ì at the Turkmen coast to record this organism and also other jelly-fishes unknown for the Caspian on videofilm. The analysis testifies that jelly-fishes refer to the Black sea Aurelia aurita, and the comb-jelly to Mnemiopsis leidyi (Ivanov etc., 2000). Thus, it is probable that the number Black Sea invaders into the Caspian is increasing.
Recently, another two species of the Black Sea origins have penetrated into epilimnion of the Caspian Sea. These species are plankton species of Copepoda: Calanipeda aquaedulcis and Acartia clausi. Invaders from the Atlantic and Mediterranean will continue appearing in the Caspian unless in next years or, probably, decade a new balance between native species and invaders is established. It is very difficult to say when such a balance is in place, because now the Caspian environment is unstable because of climate changes, anthropogenic pollution and some other important aspects of environmental impacts. Usually changes abiotic and biotic components of ecosystems of the Caspian Sea support invaders!
A positive example of invasion of strange species is the introduction of nut lotus (Nelumbo nuciferum) and water chestnut (Trapa natans). Both of the species are relicts, are of high resource and aesthetic value. Cultivation of these species on local sites of the delta of the Volga has led to further naturalisation in the interfluve of the Volga and Ural.
Bougainvillia megas (Kinn). Azov-Black sea invader, which penetrated into the Caspian Sea after opening of the Volgo-donsk channel in 1960. For the first time it was found in the gulf named after Kirov. It inhabits at small depths and participates in fouling of vessels, buoys, pipes, port structures, offshore oilrigs and piers.
The jelly-fish Blackfordia virginica (Mayer) - for the first time was discovered in the Caspian Sea at the mouth of the river Kura in 1956. Hit penetrated into the Caspian Sea through the Volgo-Donsk channel. It is often found in reservoirs with the salinity from 3 up to 18 gr/l, the optimum salinity 7-8 gr/l.
The barnacle Balanus improvisus Darvin penetrated into the Caspian Sea after opening of the Volgo-Donsk channel. For the first time was detected in the Northern and Middle Caspian in 1955, where it penetrated on the bottoms of vessels coming from the Black Sea and Sea of Azov. In a year, it distributed all over the Caspian. It is a dominant in foulings of vessels and hydraulic engineering structures.
The barnacle B. Eburneus Gould penetrated into the Caspian Sea after opening of the Volgo-Donsk channel. It is detected in the Caspian Sea in 1956. As distinct from B. Improvisus it is rarely found in fouling of vessels, since it can’t stand strong water current. It is mainly a benthic and euryhaline.
The polychaetous worms Nereis diversicolor Muller was introduced inoto the Caspian Sea in 1939-1941 from the Sea of Azov. It has successfully adapted and now occurs all over the Caspian Sea, plays an important role in fish feeding. It inhabits at the depths from 5 up to 200 ì, mainly on silty and silty-sandy grounds. It is a euryhaline species, which lives under the salinity of 0.5-36 gr/l. It can bear lowering the oxygen contents and availability of hydrogen sulphide in ground and water. Besides, it is found in vessel foulings and on hydraulic engineering structures.
The mollusk Mytilaster leneatus Gmel is an invader from the Azov-Black Sea. It appeared in the Caspian Sea in the beginning 1920-th, when small boats were delivered into the Caspian from the Black Sea from Batumi by rails. In the Caspian Sea, it was found for the first time in coastal waters of the Absheron peninsula in 1928. It is one of the leading species of benthos and periphyton in natural substrates of the Caspian Sea. It develops in great numbers in foulings of vessels and hydraulic engineering structures. It lives mainly at small depths (10-25 ì). It superseded Caspian dreysena from benthic biotic communities.
The mollusk Abra ovata is an invader from the Azov-Black Sea. It was introduced into the Caspian Sea in 1939-1940 and additionally in 1947-1948. By the end of 1959, abra populated western part of the Middle and Southern Caspian. It ranges at the depths of up to 100 ì, mainly on silty grounds, in the Middle and Southern Caspian. Abra plays an important role in cleaning water from oil by filtering 0.2-0.4 l of oily water per day.
The shrimp Palaemon adspersus Rathke is distributed in the Baltic and Black Seas, in brackish lake Adjikabul and in some fish breeding ponds (economy) of the Kura reaches mass development.
The crab Rhithropanopeus harrisii tridentatus Maitland is widespread in waters of the Atlantic coast of America, Northern, Baltic, Black, Caspian and Azov Seas. In the Caspian Sea, it was found in the vicinity of island Kulaly for the first time in 1958. From the Northern Caspian, the crab distributed with help of vessels and cyclonic current along western part of the Middle Caspian all over the sea. It occurs at the depth of 5-25 ì, and it is an euryedaphic species.
The mullet Mugil saliens Risso was intoduced into the Caspian in 1930-1934 from the Black Sea. It is widespread all over the Caspian, especially in southeastern and southwestern parts. It is a valuable commercial fish.
The mullet M. Auratus Risso was intoduced into the Caspian in 1930-1934 from the Black Sea. It is widespread all over the Caspian, especially in southern part. It is a valuable commercial fish.
The mosquito fish Gambusia affinis Boejard et Girard. It is believed that 2 species were release into the Caspian basin: G. Affinis and G. Holbrooki. In connection with this, emerging or even existence of hybrid forms is possible. This is a southamerican freshwater fish. This viviparous fish was acclimatized in Azerbaijan in 1933-1934. Nowadays, it is found from Astara in the south up to Khachmas in the northeast. It is very abundant in low-lying waters of the Lenkoran natural area, including in the Minor Kyzyl-Agaj gulf and fresher parts of the Greater gulf. Maturity occurs in 1 month, viviparous, each female bring forth 7 generations. Flexible in terms of food, however, it is not omnivorous. It was introduced to combat malaria, as a destoyer of larvae and chrysalises of malaria mosquitos. It can inflict damage by eating caviar and fish fries.
The hampback Oncorhynchus gorbusha Walbaum. 2m fries of the hampback were released into the Caspian Sea in 1964.
The eel Anguilla anguilla L. appeared in waters of Azerbaijan in 1964, when the first eel was caught in the Minor Kyzyl-Agaj gulf. In December of the same year, the second speciment was revealed in the Kura at Bogdanov’s fisheries, at the distance of about 60 kms from the mouth. Every consequent year, it was also found in coastal waters from the Sarah peninsula down to Iranian waters. Single eels used to be found in the basin of the Caspian Sea earlier. According to the data of K. F. Kessler (citation of Zenkevich, 1963), they penetrated into the basins of the Black and Caspian Seas from the Baltic. The opening of the Volgo-Baltic path and population of internal reservoirs of Russia, including basins of the Volga, with larvae eel, delivered from England and France, have facilitated its penetration into the Caspian. A rather often by-catch in different parts of the sea, provides a basis to suppose that not only single fishes have penetrated the Caspian.
Environmental problems of the Caspian Sea are multiple and various in their origin. On one hand, they are caused by the commercial use of the sea; on the other hand, human activity impacts coastal areas, including input from rivers in the Caspian. As the Caspian is an inland water body, anthropogenic (man-caused) impacts on catchment area (about 3.5 million km2) accumulate here. Anthropogenic impact on the Caspian ecosystem occurs concurrently with various natural endogenous and exogenous processes. It is primarily sea level changes, periodical seismic activity, surges and retreats, mud volcanoes and neo-tectonics. Special features of the Caspian include constant alterations of its area, volume, configuration of the coastline and water column structure. Anthropogenic activity, as well as a natural impact, can have a chronic (long term) or acute (short term) effect. Regulation of Caspian rivers discharge is an example of a long-term anthropogenic impact. Man-built dams have reduced water input into the Caspian. It took a few years to fill huge water reservoirs; during this period the Caspian lacked high amount of river fresh water. Recent rise of the level of the Caspian is an example of a long-term natural impact. It rose by 2.2 m during the period from 1997 till 1995. This natural impact on the Caspian lasted for about two years. An accident at an oil tanker can be an example of a short-term anthropogenic impact. This type of accident results in an oil spill and has a severe negative impact on a localized area. However, natural purification processes will counteract the oil spill in a short period of time. An earthquake causes a short-term natural impact on the Caspian. A few seconds of seabed movement can result in high waves, collapse of coastline and even the formation of new bays. Meanwhile, natural processes will soon erase the consequences of the earthquake.
This section describes the impact of the following factors on the biodiversity of the Caspian:
Regulation of rivers that flow into the Caspian is one of the most significant anthropogenic impacts on the biodiversity of the water body. In the 20th century, in the early 1930’s, many reservoirs were built on the Caspian rivers for the purposes of hydroelectric power industry. At present the Volga is surrounded by a chain of huge man made lakes or reservoirs (Figure 25). The capacity of the water bodies is over 180 km3. Every year 8-10 km3 of water are lost from evaporation which is approximately 3% of the annual flow of the Volga (Sonne, 2000). Besides, man made channels were built to link the Volga and then the Caspian with other rivers and with other seas via the rivers. At present the Volga links the Caspian with the oceans of the world. This great river is connected with the Baltic Sea via the Volga-Baltic waterway; with the White Sea via the North Dvina system and the Belomor-Baltic channel; with the Azov and Black Seas via the Volga-Don channel; with the Moscva river via the Moscow channel. Water from the Caspian rivers is used for irrigation which also reduces the annual flow. Water used for irrigation of fields is a loss to the Caspian. According to I.S.Sonne (2000), the annual flow of the following rivers reduced due to irrigation intake:
Volga |
9.2% |
Ural |
23% |
Terek and Sulak |
60.2% |
Kura |
12.8% |
At present the Caspian lacks about 12% of river input. During the period of water reservoir infill water loss was even higher. In 1942-45 total loss was 113 km3 or more than 8 km3 per year. In 1956-69, during the construction and operation of large hydrotechnical facilities on rivers, the Caspian lost 350 cubic kilometers of river water, or over 25 km3 per year. According to the State Institute of Hydrology, the annual loss of river input in 1956-1990 ranged from 30 to 50 km3. In 1942-1990 about 1100 km3 of river water was used for economy purposes, including 600 km3 water intake from The Volga (Bortnik et al, 1997). It was calculated that in 1930-40 water management caused 2-3 cm decrease of sea level, while in late 1970’s – early 1980’s the annual decrease reached 10-12 cm. It can be concluded that, if not for the river regulation (water intake), the level of the Caspian would have been at least 1-1.5 m above the present level (Georgievsky, Shikhlomanov, 1994). Regulation of river flow has both chronic and acute impact. Chronic impact can be described as a shoaling of river deltas. For instance, lack of river input reduced the area of delta vegetation, caused loss of reeds, cat’s tail, bushes. Loss of vegetation resulted in loss of aquatic and coastal fauna. However, not only deltas suffered the consequences of river regulation. Many anadromous and semi-migratory species were deprived of their natural spawning grounds. As river deltas became shallower, fish could not migrate to rivers for spawning. Those individuals that managed to get to the rivers, encountered the problem of hydropower plants. The impact of dam construction on sturgeon and salmon was the most severe. The species cannot overcome obstacles such as dams even with fish ways and fish lifts. The loss of natural spawning grounds resulted in almost complete loss of the Caspian salmon population; as for the sturgeon, it is bred in fish hatcheries. As per ‘The Volga revival…’, published in 1996, the annual loss of sturgeon due to hydropower plant activity is over 10,000 tonnes per year. State report ‘Status of environment of the Russian Federation in 1994-95’ contains data about the construction of dams at the Volga that resulted in the reduction of spawning grounds from 3-4 thousand hectares to 0.4 thousand hectares which is 12% of the previous grounds in the delta and food plain of the Volga and the Akhtuba. The same report highlights the loss of natural spawning grounds of beluga, Caspian inconnu, and anadromous herring. The remaining natural spawning grounds are shown in the table below.
Name |
Area of remaining spawning grounds, thousands hectares |
Kura |
0.16 |
Terek |
0.13 |
Sulak |
0.2 |
Ural |
1.4 |
The only available natural spawning grounds are located on the Ural and the Iranian rivers where no dams were built.
Accidental discharges of hydropower plants have an acute (short term) impact on the Caspian. The discharges occur in spring when reservoirs are flooded with snow water and accumulation of high amount of water is a threat to dams. To prevent this, engineers discharge a high amount of water in a jet stream. The man made floods damage bottom and coastal ecosystems and make difficult it for spring spawning migrations. On the other hand, engineers can minimize river input downstream from the dam during low-flow periods. They keep the sea level high in the reservoirs to provide continuous work for water turbines, so the riverbed downstream from the dam almost dries up. This is particularly dangerous for shallow river arms and flood plains. A chain of dry flood plains can be seen along the Caspian rivers during dry years. This tragic picture is a reminder of the tragedy of the Aral Sea which lost half of its water reserve after the input of rivers Amu-Daria and Syr-Daria had been regulated. The tragedy re-occurs in flood plains of the Caspian rivers. Fortunately, dry years are not typical for the Caspian, and such tragedies are uncommon. However, just one dry water system is enough to make unrecoverable damage to the biodiversity of the environment.
The impact of water turbines on aquatic life deserves a special mention. Entrained fish and invertebrates die or become badly damaged. Thus, every hydropower plant makes significant damage to the biological diversity of the aquatic community
Caspian rivers carry a high amount of pollutants from catchment areas produced by both industrial and agricultural anthropogenic impact. This can result in the Caspian becoming severely polluted . Fortunately, only a small proportion of diluted and suspended pollutants reach the sea, as water reservoirs serve as man made sumps or purifiers. Pollutants accumulate in bottom sediments of these huge man made lakes. If it were not for the reservoirs on the rivers, we would not be able to maintain the present the biodiversity of Caspian deltas and adjacent areas. This proves that the interface between the negative and positive anthropogenic impact is delicately balanced.
Water reservoirs on the Caspian rivers hold not only pollutants but also nutrients. The retention of chemicals that are vitally important for plant life cannot be considered as positive. Disruption of nutrient input into the Caspian significantly reduces the trophic potential of deltas and adjacent areas of the Caspian Sea and has a negative impact on its productivity and biodiversity.
The biodiversity is of a high commercial significance. Biological resources of the Caspian (mainly fish resources) are estimated as 5-6 billion USD per year (Glukhovtsev, 1997). High cost of fish resources threatens the biodiversity of the most important commercial species.
The annual catch in the Caspian (without sprat) reduced from 283 thousand tonnes in 1951-55 to 81 thousand tonnes in 1990-95. However, reduced catches of the majority of species in the Caspian cannot be related? to the reduction of resources and can be a result of an economic recession in the fishing industry. There is no real threat to the majority of commercial species.
However, fishing along with other factors (rivers regulation, pollution) resulted in the complete loss of some species of fish and Cyclostomata. In the 1920-40’s typical commercial species were Caspian lamprey, Volga shad, Caspian trout, Caspian inconnu. The total catch of these species was about 80 thousand tonnes. At present the species are included in the Red Book of Republic of Kazakhstan, Russian Federation and other Caspian states.
About 90% of world sturgeon reserves are concentrated in the Caspian, so the Sea can be considered a global genetic fund for the species. At present there is a real threat to the survival of the species. Nowadays the catch of sturgeon in the Caspian has reduced from 25,000 tonnes per year to 1,000 tonne. Before 1962 when fishing of sturgeon was legal, commercial fishing made a significant damage to the population, as many juvenile fish were caught. In late 1970’s over-fishing reached 30%.
At present fishing and criminal fishing – poaching – is the main threat to the biodiversity of sturgeon. After the Soviet Union collapsed, Fishing Regulations were not observed, fish control authorities disintegrated, marine fishing of sturgeon resumed.
Russian specialists believe that illegal fishing increases official catch by a factor of 11, i.e. by a factor of 8 offshore and three times in rivers. Fishing control authorities are not sufficiently equipped to be able to prevent poaching. The situation is complicated with what is commonly known as ‘common poaching’ when mass unemployment forced people who were previously law abiding to break the law.
The poaching problem is most severe along the Azerbaijan, Dagestan and Kalmykiya coasts. The situation in other sectors is not much better. Though the official legal catch of sturgeon in Turkmenistan is 20 tonnes per year for scientific purposes, approximate calculation based on amount of fish in Ashkabad markets gives a figure of at least 300 tonnes. significant damage to the Turkmenian population of sturgeon is made by international poachers (Iranian and Azeri vessels). At present only border guard vessels restrain this factor.
Poaching is a serious problem that must be resolved with joint efforts of the Caspian states.
The problem of over-fishing affects other species. Thus, in Iran over-fishing of Caspian trout, bream, zander, along with the damage of their habitats and spawning grounds, resulted in complete loss of these species. Zander disappeared due to massive catches in Azerbaijan and Turkmenistan.
Catches of other species is far from scientific methods and regulations, so it is not a threat and will not be a threat in the near future. Over-fishing is less dangerous for species with a short life cycle. Even if the abundance of the species reduces, it recovers within a few years under the right conditions. To recover the reserves of sturgeon with its long life cycle will take at least 30-50 years.
Caspian Sea level changes is one of the most important natural impacts on the biodiversity of this huge water body (Fir. 26, 27) (Dumont, 1995). The impact can be both chronic (long-term) and acute (short-term). A long-term impact is historical natural sea level fluctuations, which can be attributed to changes of climate and river discharge into the Caspian. Acute (short-term impact) is seasonal or wind-induced changes of level. It is known that the seasonal changes of level of the North Caspian can reach almost 0.5 m, while under the influence of surges it can rise for 1.5-2 m. At the west of the North Caspian surges cause inundation of the coastline up to 30 km onshore, while retreats cause exposure of 10 km of the seabed.
Let’s review chronic (long-term) impact of sea level changes on the biodiversity of the Caspian. In the 20th century the sea level has been decreasing from the late 1920’s - early 1930’s till 1970’s. The level of the Caspian decreased by almost 3 m. Such a significant change had a negative impact on its flora and fauna. Shallow waters of the North Caspian and deltas of Caspian rivers suffered the most. Shallow bays such as Kaidak and Mertviy Kultuk dried, the populations of the bays died. The river delta areas reduced significantly. When the sea level lowered, the exposed seabed formed new islands. Existing islands formed peninsulas or merged with the land. An example of formation of new islands on the Caspian is island Zemchuzny that appeared late in 20’s. In 1978 it was 6 km long, 0.5-1 km wide. At the beginning of the 20th century only vast shallow waters existed in place of the island. Island Cheleken at the west coast of the South Caspian is a good example of peninsula formation. In early 30-s it merged into the coast and lost the status of an island.
It is obvious, that a continuous decrease of the Caspian sea level has a negative impact on aquatic life and a positive impact on onshore organisms. Plant and animal life soon cover recently formed islands and turns them into an important component of onshore ecosystems. The above-mentioned island, Zemchuzny, is now a nesting area for waterfowl. There are also seal-rookeries of the Caspian seal on the island.
Loss of island status almost always has a negative impact on inhabitants of former island because the territory becomes more available for predators and people. This leads to reduction of the biodiversity of the island. When Cheleken island turned into a peninsula, wolves, jackals, foxes and stray dogs could easily reach the island not only via ice but also directly via dry seabed. The amount of birds and mammals on the island significantly reduced. People also visited Cheleken after it lost island status more often, and used it not only for hunting but also for cattle pastures, reed cutting etc.
Lowering of the Caspian sea level created problems for navigation. Almost all the deltas of the Caspian rivers became so shallow that dredging had to be undertaken . Grabs, suction dredges and hydraulic jets contributed to damaging the delta’s ecosystems . This activity cannot be considered a short-term impact as the equipment is used throughout the year, except for the winter period when there is no navigation. although anthropogenic activity had a negative impact on the biodiversity of the Caspian, it was also of some benefit. Dredging resulted in formation of man made islands which were occupied by animal and plant life. Conditions of the islands were the most favorable for water fowl. For instance, the extensive Volga-Caspian channel that connects The Volga delta with the navigable part of the North Caspian, is surrounded with man made islands occupied with active wild life. This channel looks like a “road” to the sea where road fencing is replaced with beautiful islands full of various animal and plant life. Such man made “roads” to the sea are typical not only for The Volga but also for other navigable rivers that flow into the North Caspian.
The above example shows that changes in the level of the Caspian Sea can have a non-direct impact on biodiversity, via human activity. Sea level changes affect human interests, and response actions can make more damage to the environment than the change itself. Construction of a dam at the head of the Kara Bogaz Gol Bay serves as an example. The Bay was blocked with a dyke in 1980. At the time over 10 km3 of water was discharged from the Caspian into the Bay, and the scientists believed that construction of the dyke would prevent the sea level decreasing to a certain extent. The result of this action was that the Bay dried within a three-year period (1981-83) and turned into a huge salt desert. Only a few percent of the previously significant original surface of the Bay was/is now covered with water. It is obvious that the drying of the Bay resulted in the death of its inhabitants such as salt-loving Crustaceans, algae and bacteria. Some of the largest species served as a food-base for flamingo, so their loss caused starvation for the beautiful birds. As with the flamingoes, humans also suffered from the construction of the dyke at Kara Bogaz Gol. Nauplii (cysts) of salt-loving Crustacean Artemia salina were used for fisheries and aquarium fish breeding as a food source for juvenile fish. As this type of food is highly required all over the world, there was a branch of industry to prepare the cysts for sale. When Artemia salina disappeared, this branch of the industry collapsed. Minerals production that was historically typical for the area also suffered the consequences of the Kara Bogaz Gol tragedy. According to L.A.Zenkevich (1963), the waters of the Bay contained about 18 billion tonnes of mineral salts including 9.3 billion ton of sodium chloride, 5.3 billion ton of magnesium sulfate, 2.8 billion tonnes of magnesium chloride. In winter, when the water temperature in Kara Bogaz Gol decreased, about 8 billion tonnes of mirabilite settled in sediments of the Bay. People use these minerals, and production of the minerals was commercially important. . Therefore the failure of mineral production at Kara Bogaz Gol due to the drying of the Bay had a significant impact on the economy of this Caspian region.
It is clear that construction of the dyke in the head of the Kara Bogaz Gol Bay had a negative impact on both the biodiversity and economical activity of the area. In 1992 the dyke was totally destroyed, and within 9 years the Bay became partially rehabilitated. A population of A. salina in the Bay rehabilitated. Therefore flamingo returned to Kara Bogaz Gol. Production of cysts of Artemia salina also re-commenced. After Turkmenistan claimed its independence in the middle of 1990’s, a Turkmen-Belgian company specializing in producing cysts of Artemia salina was founded in the area. The Company is operating successfully and profitably (Atamuradov, 1999). Production of mineral at Kara Bogaz Gol is also successful again. Not only is the surface and the volume of the Bay restored but also its biodiversity. In addition to A. salina a salt-loving Crustacean Molina mongolica was found in the Bay. In the shallow waters of Kara Bogaz Gol salt loving water larvae of flies and mosquitoes were found. Water of the Bay is full of bacteria and algae. The most abundant are algae Aphanothece salina that form multiple mucous colonies at the coast, and flagellates Dunaliella viridis and D.salina that bloom during the period of mirabilite settlement. 1 g of firm salt contains about 0.5 million of Dunaliella cells, 1 cm3 of water contains 21 million of bacteria in average. Thus, re-establishing the natural discharge of the Caspian water in Kara Bogaz Gol allowed the biodiversity of the bay to rehabilitate.
Chronic (long-term) impact of sea level rise is xerophytization of coastal plants and desertification of coastal areas.
Let’s discuss a chronic (long-term) impact of the sea level rise on the biodiversity of the Caspian. In the 20th century, the sea level has been rising from 1978 untill approximately 1996. The total rise was about 2.5 m. This had a negative impact both on the biodiversity and economical activity. Sudden level rise damaged industrial, agricultural facilities and inhabited buildings located at the coast. A lot of pollutants were discharged into the sea which reduced populations and even resulted in localized death of animal and plant communities. First of all, flooding of oil production and transportation facilities had a damaging effect on the biodiversity. Many were surrounded by localized oil spills that polluted sea water after the rise occurred. The west coast of the North Caspian suffered the most where many economical facilities were flooded (Atirau and Mangistau areas of Kazakhstan). In the Atirau area the coastline moved 70 km onshore. About 1 million hectare of land was flooded, most of it was previously used for agricultural purposes. According to Ozturk and other authors (Ozturk et al., 1999), total economy damage was about 150,000,000 USD. Oil and gas fields were most threatened . The Tajigali, Pribereznoe, Pustinnoe, Morskoe, Terenozek, Ugo-Zapadnoe fields and others were flooded. There is a possibility that the Kalamkas and Karazanbas fields in Buzachy district of Mangistau area will be flooded. These fields provide 50% of the oil for the area. The Arman, Zalgistob and Severny Buzachy fields located in the same area were completely flooded. Many wells were drilled during a few decades of oil production in these areas. The exact number of the wells is not known. According to I.S.Sonne (2000) there is enough oil in any operating, suspended or abandoned well to cause a local ecocatastrophe, to destroy spawning grounds of fish, nesting grounds of birds and seal-rookeries of Caspian seals. Onshore well equipment was not covered with underseal so it would get corroded under water. Oil equipment can be cut with ice movement in winter and spring resulting in oil spills. Cement plugs of suspended onshore wells would be destroyed under water also resulting in oil spills. We believe that the pollution of the North Caspian from flooded suspended onshore wells would be several times higher than the pollution from the existing wells. Therefore previous oil fields are a bigger threat to the sea than the present ones.
A continuous sea level rise made significant damage to plant life in deltas of Caspian rivers. Trees cannot be constantly flooded, so dead forests can be found in all river deltas. Fast sea level rise for more than 2 m within less than 20 years had a negative impact on m any plant communities; some of them were lost due to flooding. Animal communities also disappeared with the loss island. However, we believe this was not such a tragedy as other representatives of the species managed to survive on non-flooded islands.
The positive impacts of long-term sea level rise are the improvement of spawning ground conditions, increased spawning ground areas, reinforced water exchange between different sections of the sea, extension fresh water of buffer zone and increase of potential productivity in the North Caspian.
Consider the short-term (acute) impact of level changes on biodiversity of the Caspian. As mentioned above, these are seasonal fluctuations or surges and retreats. As a rule, short-term sea level changes do not have any significant impact on biodiversity of the Caspian but slightly decrease abundance of individual species of onshore animals and do not affect the abundance of plant life. Of course seasonal fluctuations or surges can cause death to some animals or even people but the percentage is insignificant. Temporary flooded onshore plants do not suffer such consequences and easily recover. Impact of seasonal decreases of sea level or retreats is also minimal. As a rule, only some fish die on the exposed seabed, whole invertebrates and plants easily survive the unfavorable conditions. The most tolerant to surges and retreats and seasonal fluctuations of sea level is the reed Phragmites australis. This plant is wide spread in Caspian deltas. They form communities that can be found up to 20-30 km offshore and at 2-2.5 m depth.
It can be concluded that sea level rise does not allow endemic Caspian aquatic life to spread to the north due to desalination of the Caspian. When sea level reduces, its salinity increases and the abundance of true Caspian endemics increases.
Pollution is a significant threat to the biodiversity of the Caspian. The sources of pollution are industrial, agricultural and accidental discharges and sewage. The main flow of pollution comes from Volga. The Volga input contains discharges from other sources that did not accumulate in reservoirs and its delta. The Volga discharges are comparable with oil field and industrial discharge from the Baku and Sumgait facilities and with the Kura discharge. In Turkmenistan the main pollution comes from the Turkmenbashi oil refinery and from the Cheleken oil fields. The input of Kazakhstan into the pollution is not so significant. Pollutants mainly come from flooded oil fields and with the Ural river discharge.
The highest level of pollution was observed in late 1980’s. Later input of pollutants into the sea reduced due to economic crisis, reduction of industrial capacity and abandonment of plants.
The most typical toxicants in the Caspian are petroleum hydrocarbons, heavy metals, phenol, surfactants, chloral-organic pesticides. Oil pollution is the most dangerous one. Interaction of aquatic life with petroleum hydrocarbons causes various physiological, biochemical and morphological changes in organisms. In some cases the changes can be reversible, otherwise they cause chronic pathological effects that result in death of fish.
Oil pollution of the North Caspian ranges from 1 to 6 MPC. The largest input in oil pollution was made by oil production that commenced in late 1940’s at Apsheron peninsula, and later – at Cheleken peninsula. In 1980’s exploration of the Kalamkas, Karajambas and Tengiz fields commenced in the north coastal waters of Kazakhstan. As the fields are located in shallow waters and protected with dams that were constructed with light soils, they regularly get washed out and carry pollutants from the fields to the sea. The sources of oil pollution of the Caspian are primarily offshore wells during the drilling and production phases. (oil outflow on the surface of the sea) formed in 27 wells during the exploration of Oily Rocks gryphons. Some of the gryphons’ activity lasted from a few days to 2 years. The amount of oil coming out of the gryphons was about 100-500 tonnes per day (Mailyan, 1966).
The following events can also result in oil pollution of the sea: underground and underwater maintenance of operating wells, accidental pipeline breaking, cleaning of effluent of oil refineries. Till recent developments, air surveys carried out by GOIN to control oil pollution in the surface layers, regularly registered large drifting oil spills (hundreds and thousands of square kilometers).
Though oil toxicity (A.Nelson-Smith, 1977) is considered less dangerous for aquatic life than the toxicity of heavy metals, pesticides and some other organic pollutants, its impact on marine life must not be underestimated. Recent data from continuous experiments and the most sensitive indicators confirm that even low oil concentrations (below MPC) have a toxic effect. Toxic impact on fish is identifiable even at relatively low concentrations (0.0.1-0.1 mg/l). This impact causes not the death of fish but deterioration of their physiological condition, feeding, reproduction and other life processes. Higher oil concentrations (up to 15 MPC) have a significant impact on fish. This and higher concentrations of crude oil and its derivatives reduce growth and development rate, fertility, reproduction capacity (Abbasov et al, 1991; Kasymov et al, 1992). Fertility of females of every next generation decreases a few times. If in first generation it reduces for 10%, in second and third it reduces for 25-30% respectively. Reduction of reduce growth and development rate, fertility, reproduction capacity is caused not only by direct impact of oil on gonads but also by significant deterioration of physical conditions of the fish. Besides, it impacts immune system, first of all, leucocytes. That increases possibility of infecting fish with various diseases and induces cancerous growth; oil has carcinogenic compounds. There is information in literature about teratogenic effect (growth of cells unusual for the organism) and new growth in organism of sturgeon (Romanov, Altufiev, 1990).
Many scientists outline negative impact of drilling muds and stratal water on fish. Long term impact of the above compounds causes quantitative changes in basic blood elements, raise of mortality rate of fish larvae, reduction of activity and orientation (Gorbunov, 1989; Isuyev, Gabibov, 1989).
The second common toxicants are heavy metals. The most dangerous for biocenosis elements are lead, cadmium, zinc, and copper. Trace metals that accumulate in liver and gonads induce changes of the organs, and depress immune functions of organism. The highest concentrations of trace metals are typical for predators: catfish, zander.
In 1990’s concentrations of copper and zinc increased compared to early 1980’s, and exceeded maximum permissible concentrations 5 times. In contrast, average concentrations of lead that ranged from 1.2 to 5.7 MPC previously in 1994-96, were at MPC level.
Concentration of copper in fish tissue ranged from 0.12 to 2 MPC; the highest concentrations were found in liver and gonads of sturgeon and Stellate sturgeon. High concentrations of cadmium were found in the same organs. Zinc content in tissue of sturgeon ranged from 6.0 mg/kg to 70 mg/kg of wet weight.
In 1994-96 mercury concentrations in muscle tissue of sturgeon slightly increased. Thus, in 1993 the average value was 0.048 mg/kg, in 1994-96 they ranged from 0.10-0.19 mg/ kg of wet weight.
Pollution level varies in different sections of the sea.
The Volga water is described as ‘medium polluted’ with transition to ‘very’ and ‘highly polluted’. Ecosystem is generally described as affected by anthropogenic pollution with elements of environmental regress.
General toxicity of water at the same level of pollution varies highly and depends on ecosystem condition (hydrochemistry, eutrophication etc.). Toxicity of water of Volga delta assessed by means of biotesting and general toxicity range varied from low toxic to high toxic depending on the time of year and part of delta. Biotesting was being carried out during 127 days (April-September). At spawning grounds toxic affect of water on juvenile fish increased with no relation to its quality in the river. Besides, it had general toxic and genotoxic and embryotoxic effects.
Anthropogenic pollution of The Volga delta makes regular damage to commercial resources of the Volga and the Caspian. Preliminary estimate of damage made by toxicosis to fisheries of Volga-Caspian area gives a figure of 8,000 of semi-migratory and fresh water fish. 13.7-89.3% of Caspian roach, bream, carp, silver bream, blue bream, perch and zander in delta of the Volga had symptoms of toxic poisoning prior to spawning.
Water of the Middle and South Caspian is described as medium-polluted or polluted. As a result, the biodiversity of benthic fauna reduced by a factor of 3 to 10. In the Sumgait area and Baku Bay the abundance of Crustacean and molluscs reduced. The same happened at Kura delta. The environmental situation in the Baku Bay is catastrophic. The seabed is covered with domestic waste, oil products, heavy metals and organic compounds; no seabed benthic fauna is present. Complex assessment of environmental situation in the Azerbaijan sector of the Caspian identifies water of the sector as polluted to high polluted by microbiological and hydrochemical indicators.
During last 6 years concentration of oil products in coastal waters of the Krasnovodsky Bay tend to reduce; concentration of phenol reduced from 7 to 1.5 MPC. However, this is a result not of a technology update, but of the cancellation of drilling operations and general activity reduction.
In Turkmenistan sector of the Caspian the most polluted areas are coastal waters of the Krasnovodsky Bay and Cheleken peninsula, where average annual concentration is 2-4 MPC. The pollution is mainly related to Turkmenbashi oil refinery and transfer terminal, marine transport, and operation of oil wells.
Previously high level of pollution was recorded also in Ogurchinski and Kuuli Cape.
Increase of anthropogenic stress in the ecosystem of the Caspian (pollution with pesticides, oil products, heavy metals) had primarily impacted sturgeon. It caused a disease of sturgeon not recorded previously which is gepatoxic hypoxia, the symptom of which is exfoliation of muscle tissue.
Chloral-organic pesticides that were widely used in agriculture, health protection and other activity in 1960-80, had a major impact on pathology of fish. As per Russian specialists, during this period the most massive affection of sturgeon occurred. Almost all forms of metabolism were upset including protein, carbohydrate, lipid and mineral metabolisms. In kidneys albumen dystrophy, chronic nephritis were found; in liver – albumen and fat dystrophy, necrobiosis of hepatocytes, cirrhosis. A pathology of spleen and muscle tissue was recorded (The Caspian Sea, 1996; Belyaeva et al, 1998).
Functional errors in work of various systems and organs upset reproductive capacity of sturgeon. The errors included growth of hermaphrodites, growths in gonads, ovocestice, deterioration of gamete capsule. At the end of 1980’s 44-51% of spawning population of sturgeon had upset reproductive function (Belyaeva et al, 1998).
Study of affection of muscle tissue of sturgeon (as the most abundant marine species) and measure of pesticides and oil products concentration in different sectors of the Caspian Sea showed:
Study of physiological and biological condition of sturgeon in 1990’s revealed some decrease of pathologies compared to the level of 1960-70’s. However, there was no return to relatively normal conditions. The toxicosis has become chronic with periods of improvement and acute condition. During the period of improvement the amount of fish with significant functional and morphological pathologies reduced. Some of the studied physiological, biochemical and morphofunctional parameters tend to improve, even to return to relatively normal condition. The period of 1994-96 can be described as a ‘phase of unstable functioning of physiological systems’.
At present, changes in organism of sturgeon have not become irreversible. There is information that confirms rehabilitation of one of the systems of immune function (antioxidant) of sturgeon. This is the basis for rehabilitation of other functions and systems of organism.
A significant toxic impact was made on seals. Recently recorded pathology of the Caspian seals is a very complicated combined process that is classified as cumulative politoxicosis caused primarily by pollution. Heavy metals mainly accumulated in liver and hypodermic fat (Khuraskin et al, 1994).
Studies of 1994-95 showed that compare to 1993 toxicological indicators reduced. Content of toxicants in tissue of adult seals did not actually change which is due to very slow degradation of DDT. Unlike in 1993, none of the samples contained heptachlor and aldrin. Heavy metals are second important toxicological stress index. Content of mercury in tissue of adult species reduced by a factor of 5.8 compare to 1992, content of lead reduced by a factor of 11.5. Accumulation of heavy metals in pups compare to previous years also has trend to reduce. Thus, average concentrations of lead (in liver) reduced from 3.1 to 0.5 mg/kg, concentrations of cadmium – from 1.3 to 0.14 mg/kg of wet weight, concentration of mercury did not change significantly.
The surveys confirmed a hypothesis of Japanese environmentalist S.Tanabe (1986) that original disbalance of pups occurs during pre- and postnatal periods of development as pups get it with mother’s milk. The most clear negative connection of female and the pup is recorded during transfer of chloro-organic pesticides. Insignificant transfer of heavy metal occurs through milking.
In general, it can be concluded that pollution is not a leading factor in formation of main biological productivity of the Caspian. At the same time, this factor is determining for localized areas where pollution is continuous, like polluted areas in the south oil fields. Ecological situation along the west coast of the Middle and South Caspian is unfavorable, ecological situation in Baku Bay is critical.
Development of processes of eutrophication and pollution in fresh and saline waters causes reduction of fish reproduction. As estimated by specialists, the catch of semi-migratory fish in Volga-Caspian region (Caspian roach, bream, zander) would reduce for 60% due to three factors: regulation of rivers discharge, pollution and eutrophication of waters.
Therefore, in spite of a certain negative impact of pollution on condition of ecosystems, its effect is limited with the level of population, as it deteriorates the quality of the environmental and level of possible reproduction and abundance but does not affect the biological diversity itself. Probably these factors affect the biodiversity at genetic level but not at species level. This is the most realistic assessment confirmed by the practice.
The impact of introduced species on the biological diversity of the Caspian Sea falls into two groups: chronic (long term) or acute (short term) impact. Acute impact is identified during first years after the introduction of the new species into the Caspian. Its positive or negative impact is highlighted most clearly during these years. Later the ecosystem adapts to the introduced species, and its positive or negative effect weakens while its impact on the biodiversity becomes chronic (long-term).
All present resident species in the Caspian can be described as introduced. The only difference is the time of introduction. Some of the species were introduced so long ago that now can be considered 100% resident species.
Aquatic organisms of the Caspian can be divided into four groups. The first group is the most ancient introduced species. A scientific name for them is indigenous. Their ancestors lived 20,000,000-30,000,000 years ago and were the descendants of the ParaThetis inhabitants. As the ParaThetis was a huge northern bay of the ancient ocean Thetis, all aquatic life was introduced from thetis. Thus, Caspian indigenous species are the descendants of ancient introduced organisms from the presently non-existing Thetis. Therefore indigenous Caspian species are called ‘living fossils’.
The second group is Arctic introduced species. A detailed list of the species is given in Section 3.4.5. A scientific name of the species is glacious relicts. The ancestors of the species were introduced into the Caspian 1,000,000-1,500,000 years ago during the period of melting of a huge ice sheet that covered almost all Europe, Arctic and coastal areas of the Baltic and the White Seas. The northern species reached the Caspian with melted waters. There are several opinions about the way the species reached the Caspian that were reviewed in previous Sections. We would like to review one more way proposed by Grosswald (Grosswald, 1980) and Dawson (Dawson, 1992) (Figure 28). The scientists believe that a superflood occurred during the late Valdai period. The level of the ancient Caspian rose by 2-3 m above the level of the oceans of the world (Lamb, 1977), and its waters run through the Azov-Black Sea basin. Waters of a large ice lake that existed in the West Siberian Plain run into Aral basin and from Aral into the ancient Caspian.
The third group includes introduced species from the Black and Mediterranean Seas. Their scientific name is ‘Atlantic introduced species’. The most ancient of the species were introduced into the Caspian 50,000 years ago during Khvalyn period. The ancient Caspian was then connected with Azov-Black Sea basin through the Manych channel. Seven species were introduced into the Caspian in a natural way including Zostera nana, Cardium edule, Fabricia sabella, Atherina mochon pontica, Syngnathus nigrolineatus, Pomatoschistus caucasicus, Bowerbankia imbricata (Zenkevich, 1963). Some scientists (Fedorov, 1958; Fedorovich, 1987; Starobogatov, 1994) deny natural introduction of the species into the Caspian, as they believe that strong current in the Manych channel had always been directed away from the Caspian. If this point of view is correct, 50,000 years ago a first anthropogenic impact on the biodiversity of the Caspian was recorded.
In the 20th century the amount of the introduced species from the Black and the Mediterranean Seas suddenly increased. All the cases of introduction were related to anthropogenic activity. In 1920’s 4 species were accidentally introduced into the Caspian: algae Rhizosolenia calcar-avis, bivalve Mytilaster lineatus, and two species of shrimps: Leander squilla and L. adspersus. There is no reliable information about the way the species were introduced. Scientists suggest that merchants, who transported their small wooden boats on carts from the Azov Sea to the Caspian, could introduce them. The four species could be introduced with Azov water that remained in the boats, or in cages with living fish. During the first years following introduction the abundance of the species was quite high; they suppressed the Caspian species. For instance, in 1936 the biomass of algae Rhizosolenia calcar-avis was several millions tonnes which was about 65% of the total plankton biomass. Following the ‘biological wave’, the abundance of the species reduced; an acute phase of impact on the biodiversity of the Caspian turned into a chronic one. A short raise of abundance and further reduction was recorded for bivalve Mytilaster lineatus, and the two species of shrimps. Later people deliberately introduced five more species into the Caspian. These included two species of mullet (Mugil auratus, M. saliens) (Mugil auratus, M. saliens), one species of flounder (Pleuronectes flesus luscus), one species of Polychaeta (Nereis diversicolor) and one species of bivalves (Abra ovata). All the species adapted to the conditions of the Sea, and after a first abrupt raise of abundance stabilized and became a part of the ecosystem of the Caspian. In the middle of the 20th century, after the Volga-Don channel has been built, a new group of species was introduced into the Caspian. Some of them were introduced in ballast water of vessels, others were attached to the bottom of vessels. The following species were introduced with ballast waters: plankton Crustacean (Pleopis polyphemoides), jelly fish (Blackfordia virginica), four species of algae (Ceramium diaphanum, C. tenuissimum, Ectocarpus confervoides f. fluviatilis, Polysiphonia variegata) and crab (Rhithropanopeus harrisi). The following species were introduced with biofouling: sea acorns (Balanus impovisus è B. eburneus) and one species of pearlwort (Membranipora crustulenta). Only barnacles had a raise of abundance, it was not so obvious for other introduced species. Introduction of Atlantic species into the Caspian through the Volga-Don channel continues. A full list of recent introduced species is given in Section 3.4.2 where main groups of species of the Caspian were listed. We would like to mention only two plankton species (Copepoda aquaedulcis, Acartia clausi) and Ctenophore (Mnemiopsis leidyi), that were introduced into the Caspian with ballast waters at the end of the 20th century. The first two species can be an example of a positive introduction as they are used as a food base by plankton-feeding fish and increase the value of the Caspian zooplankton. As for the Ctenophore, this species is an example of a negative impact on the biodiversity of the Caspian (Ivanov et al, 2000). The species eats out zooplankton and causes starvation for the plankton-feeding fish. There is an opinion that the Ctenophore can cause complete loss of Caspian population of sprat (Aladin, Plotnikov, 2000). If this happens, the Caspian seal will also be lost.
The fourth group includes species introduced from fresh waters. As the above-mentioned species, they were introduced into the Caspian long ago, so they can be divided into ancient and recent species. The most ancient introduced species are Caspian Gastropods that were originated from fresh waters of Pliocene. They could have been introduced into the ancient Caspian approximately 2,000,000-5,000,000 years ago when it was the most desalinated. This could explain the relation of the species to Baikal molluscs. It is also possible that Caspian Polychaeta (Manayunkia caspia) was also introduced into the Caspian from fresh waters of Pliocene several million years ago. Caspian Carp was originated from the more recent introduced species. It was probably introduced into the Caspian during recent post-ice transgression, when the Sea received a lot of water from the rivers. Fresh water species could have been introduced during Khazar transgression (400,000 ears ago), or during Khvalyn transgression (50,000 year ago) or New Caspian transgression (5,000 years ago). There is no doubt that introduction of fresh water species into the Caspian continues nowadays. However, it is difficult to register the species so no attention is paid to them. This conclusion can be confirmed by works of U.S.Chuikov (1996, 2000). He found many new microscopical fresh water species in the Volga delta and the adjacent coastal waters of the North Caspian. The species included Rotifera, Cladocera and Copepoda Crustaceans. There were no previous surveys of the fresh water species introduced into the Caspian.
The distribution of the above four main groups of the Caspian species varies with the different sections of the Caspian (Figure 29). Thus, 75% of species in the Middle and South Caspian are Caspian indigenous organisms, 20% are fresh water species, 3% are Atlantic introduced species, and 2% are Arctic species. A proportion of the species in the North Caspian is different. Fresh water species dominate here. The proportion of them is 60%, Caspian indigenous species are 36%, Atlantic species are 4%, Arctic species – less than 1%.
No above-mentioned species can be found in the Kara Bogaz Gol Bay. Only salt-loving species (halophyles) inhabit the area. They represent another group of introduced species in the Caspian that originated from cosmopolitan forms of arid areas. All salt-loving organisms have a resting stage that can survive drying, freezing and other unfavorable conditions (Makrushin, 1985). The resting stage can be delitescence eggs, spores, seeds and cysts. They develop in saline water. The resting stages are small and can be carried with wind and migrating birds. Some cysts remain viable during tens and hundreds years. Thus, when in early 1980’s the Kara Bogaz Gol Bay dried, resting phase of salt-loving organisms remained on its exposed seabed and revived after the Bay was refilled with salt water. We would like to point out that the dry Kara Bogaz Gol Bay was a source of many dust storms. The storms carried the cysts all over the world. This is the reason why all salt-loving organisms are considered cosmopolitan. Therefore, many of the salt-loving organisms that inhabit the Kara Bogaz Gol Bay are similar to the species from slat lakes of America, Europe, Asia, Africa and Australia. It is also worth mentioning that these forms are the most ancient inhabitants of the Earth that did not really change during several hundred million years. Some scientists (Clegg et al., 1997; Lee, 2001) believe that resting stages of some halophyles can travel between planets in meteorites, remaining vital.
Consider a role of introduced species in the ecosystem of the Caspian and their impact on its biodiversity. We believe that to dramatize the impact of introduction is not right. As highlighted above, the present community of the Caspian contains mainly introduced species that form its rich biodiversity. However, the Caspian is not open to any exotic species that get into it. There is no doubt that such introduced species as Ctenophore needs to be neutralized. The surveyors of the Caspian should have balanced and differentiated approach to the problem of introduced species in the Caspian.
There is no doubt that the most ancient introduced species in the Caspian should be protected against any negative impact, including the impact of new introduced species. The Caspian indigenous flora and fauna are the main value of this continental water body. These living fossils are of high biological, ecological, genetic and commercial importance. Some surveyors believe (Dumont, 1998; Sonne, 2000; Aladin, Plotnikov, 2000) that biological resources of the Caspian are more valuable than its oil and gas resources. We would like to outline that the most valuable fish is Caspian indigenous sturgeon that is the descendant of inhabitants of the ancient ocean Thetis.
We are positive about aimed introduction of species into the Caspian. Introduction of Polychaeta Nereis and bivalve Abra were done as recommended by the scientists (Karpevich, 1975). It significantly increased value of the Caspian benthos as a food base. Adaptation of mullet and flounder was also successful. There can more perspective introduced species in the area. However, search for the species should be based on scientific studies; any haste must be avoided. The opinion to reject any introduction of the species into the Caspian is not correct. Due to specifics of its formation, this great lake has free econiches that can be filled by people. New aimed introductions in the Caspian should not put us off. However, successful ecosystem management of this water body is possible only on the basis of long-term and well-financed surveys.
A range of accidental introductions appeared to be commercially useful. Many microscopic Crustaceans increased the value of the Caspian plankton as a food base, shrimps increased the value of benthos. A high amount of accidental introduction did not have any significant impact on the biodiversity of the water body. However, some of them such as balanus and bivalve Mytilaster had a certain negative impact from people’s point of view. The abovementioned organisms cannot be used as food for fish because of their thick shells; they are typical representatives of non-food benthos. Besides, the introduced species can inhabit bottoms of vessels, port piles and offshore oil rigs. They hamper movement of the vessels and gradually corrode port and oil structures.
As a conclusion, we would like to outline that it is necessary to develop a set of measures to protect the biodiversity of the Caspian and its ecosystem against the most recent introduced Ctenophore species. This is probably the most dangerous introduced species in the Caspian Sea. We have already mentioned that the species eats zooplankton and causes starvation for other plankton-feeding species. In 1980’s it was introduced onto the Black sea and made an unrecoverable damage to its fish reserves (Ctenophore…, 20000). By late 1980’s the total weight of the Ctenophore was about billion tonnes. This highly productive organism ate not only zooplankton but also caviar and small larvae. Later the Ctenophore was introduced to the Azov sea via the Kerch channel where also made unrecoverable damage to the commercial fishing. At present it is in the Caspian, and we should prevent the tragedy of the Black and Azov Seas. We propose to commence a continuous monitoring of the species distribution in the Caspian. We need to find natural predator of the Ctenophore and after a range of laboratory and natural condition tests introduce it into the Caspian. We also believe that it is necessary to have a strict control of ballast water to avoid any accidental introduction.
The impact of climate changes on the biological diversity of the Caspian is not well studied. The majority of the surveys relate to the field paleontology. In other words, these are the studies of ancient climate changes impact on fossils that used to inhabit water bodies that were in the place of the Caspian. The majority of the scientists believe that climate changes impact on the biodiversity of the ancient Caspian was indirect, through climate impact on the sea level and its salinity. Climate changes induced the sea level and salinity changes of the ancient Caspian. These changes significantly altered the biodiversity of the water body. Nowadays we know (Rodionov, 1994) that fall of temperature causes the sea level raise, while rise of temperature causes decrease of the sea level and increase of salinity. Thus, during the period of transgressions at the Caspian, fresh-water and originally fresh-water species dominated, while the abundance of marine salt-loving species reduced. Marine species would only survive in the most saline parts of the ancient Caspian. During regression the situation was the opposite. Salt-loving species used to dominate while fresh-water species would survive only in deltas and adjacent areas of ancient Caspian rivers. There were periods in paleolimnological history of the Caspian when sudden and severe changes of climate caused such significant changes of the seal level, salinity and temperature that many ancient species were lost. It is worth mentioning though, that such catastrophes did not occur often, and some species managed to survive and to rehabilitate the biodiversity during the process of evolution. Thus, the evolutionary line of the Caspian indigenous species never broke in the Caspian. Therefore so many living fossils inhabit this unique water body.
At present climate changes also have their indirect impact (through the sea level changes and salinity) on the biodiversity of the Caspian. Of course, this impact is very weak and less obvious than the above-mentioned examples of climate changes impact on the biodiversity during the previous geological periods. It is more convenient of paleontologists, rather than zoologists and botanists, to study this process, as their studies are based on paleontological chronicles, i.e. fossils, where time is pressed and the impact of climate changes is clear. The specific feature of the 20th century is an anthropogenic impact on global warming. As many scientists believe (Hansen et al., 1988; Schneider, 1990; Kellogg, 1991), there is a possibility of temperature increase all over the planet due to ‘greenhouse effect’. These expectations are based on General Circulation Model, and on double increase of carbon dioxide concentrations in the atmosphere (Rodionov, 1994). There is no doubt that global warming is a reality of the 20th century and a new coming millennium. The scientists cannot agree about the speed of the global process. Some of them believe (Kerr, 1989; Budyko, 1991) that since 1975 till 1989 the temperature increased for more than 0.30C, and the reason was anthropogenic impact. 1980’s was the hottest decade all over the world, 1990 was the hottest during the period of observations that commenced at the end of the 19th century.
However, this opinion does not agree with the Caspian Sea level raise. As mentioned above, its level has a negative association with the temperature. Till 1978 decrease of the sea level confirm the hypothesis of global warming. However, later a sudden raise was contradicting to the conception. The event on the Caspian make us doubt in gradual global warming and continuous temperature increase. The sudden sea level raise at the end of the 20th century induced suggestions that the global warming process is more complicated and non-linear, and the general anthropogenic warming can have unstable periods and even cooling (Rodionov, 1994).
Some scientists (Klige, Myagkov, 1992; Budyko et al., 1988) carried out special calculations that show that global anthropogenic warming must cause reduction of the sea level. As they believe, even at an early stage of global warming cyclones will carry more rains into the Caspian Sea that will increase the Caspian rivers input. However, the increase of the global temperature should increase evaporation which would not only compensate increase of rivers discharge but even will exceed it. Klige and Myagkov (Klige, Myagkov, 1992) calculated that global warming of 2-3 degrees will cause increase of the total annual Caspian rivers’ discharge for 50-60 km3. Precipitation will increase for 25-30 km3. The annual evaporation will exceed the p resent evaporation fro 100-115 km3. As a result, the global warming must cause the reduction of the sea level by more than 4 m. If the calculations are correct, and if the conception of global anthropogenic warming is right, a sudden decrease of the level of the Caspian should be expected in the nearest future which will impact its biodiversity (see Section 4.4).
The history of classical scientific studies of the Caspian covers over three centuries. Although scientists from all Caspian countries participated in them, it was the Russian Empire and the ex-USSR scholars who have contributed the most. Below we try to support this statement with the synchronism of historical events around the Caspian (Sonne, 2000). We shall analyze only those most important of them, which influenced the study of the physical parameters of this water body and its biological diversity.
In XVII-XVIII, the first sufficiently accurate map of the Caspian appeared as the result of the five-year work by the German captain Jeremy Mayer, which was invited to Russia into the employ of Peter I. The map was issued in 1704 and was different from the one made by the Astronomer Royal J. De Lille in Paris in 1700. The latter showed the width of the Caspian equal to its length, and the Oxus River (Amu-Dariya) to flow in the Caspian and not the Aral Sea. Besides, the eastern coast was outlined drastically incorrect.
During the reign of Peter I, the preservation of Caspian fishery was prioritized. The late 1704, a special royal decree ordered to transfer the Astrakhan fisheries to the chest and put them under the supervision of the specific Fish Office established by Peter’s order. To implement this royal decree, dedicated work force from the inland was brought to work in the fisheries of Astrakhan.
In 1715 the first expedition of A. Berkowitch–Cherkassky to the Caspian was held during which the entire eastern coast up to the South Astrabad Bay was fully described and the fact of the Oxus River (Amu-Dariya) not flowing in the Caspian was proved again. The map based on the findings of this expedition was much more precise than the one produced by Jeremy Mayer. A year later the second expedition along the Eastern coast was held, which resulted in establishing two fortresses such as Fort Shevchenko on the Tube-Karagan peninsula, Saint Peter’s Fortress in the Alexanderbay Gulf, and the one on the Krasnovodsk spit of the Mangyshlak peninsula. Later in 19th century the town of Krasnovodsk emerged at this place.
In 1717 Peter I visited France. Among his intentions was a meeting with J. De Lille, when the czar assured him that the Oxus River (Amu-Dariya) did not flow in the Caspian. Also, the Russian czar offered him the map made per the findings of Berkovitch-Cherkessky expedition, which displayed a big bay at the Eastern Coast that was named Kara Bogaz Gol later on.
In 1719 Peter I arranged a new expedition led by the lieutenant commander Van Verden with the objective to describe the west coast from Astrakhan up to the Kura River. A year later the head of the expedition together with F.I. Soymonov continued their work on the south coast of the Caspian. Based on findings of this expedition, a new map named “The Flat Map of the Caspian from Yarkovsky Outfall to the Astrabad Bay, longitude given in degrees and minutes, depth given in sagenes and feet”. This map that was the first to show the real dimensions and correct outlines of the lake and looked much alike the existing modern maps. In 1721 Peter I, being the honorable member of French Academy of Sciences, ordered to send this map to Paris, where it was recognized as a world-range scientific sensation.
During all the above-mentioned expeditions, cartographic and geographic observation was held along with primary geological, zoological, botanic and ethnographic studies. All the findings were sorted and transferred to the custody of Russian Imperial Fleet archives. But with the establishment of Russian Imperial Academy of Sciences in 1724 a part of these records was offered under the competence of the Academy.
Russian Empire was not the only one to take interest in the Caspian studies, and the British Empire’s attempts to intensively research the lake should be mentioned. Thus, Historical Report of British Trade Company on the Caspian in two volumes totaling 700 pages by Jonas Henway was issued in London, in 1753.
In the late XVII (1768-1774), the first expeditions of Russian Imperial Academy of Sciences to the Caspian were held. These expeditions headed by Academicians Gmelin and Palass gave primary valuable knowledge of the Caspian in terms of historical geology and biology. These years may be considered the beginning of the biological diversity studies of the Caspian.
In 1781 another survey led by M.I. Voynovich was sent to the Caspian area, during which the Astrabad and Krasnovodsk Bays and Dervish and Cheleken Islands were described. At the same time, they studied biological resources of these bays and islands and first supposed the deposition of oil in the Caspian subsurface. Also, Voynovich was the first to note the fluctuation of the Caspian level.
In the early 19, the Admiralty Department of the Russian Empire charged to 12-class navigator A.E.Kolodkin to make a comprehensive atlas of the Caspian Sea. 18 year later in 1826 he published the detailed atlas of the Caspian consisting of 17 maps.
In 1825 naturalist E.I.Eichwald undertook an expedition to study the Caspian Sea and its coastal fauna. A famous surveyor, he was the first to study the biological diversity of the sea along the coast. His works on Cheleken Island, Krasnovodsk Bay and Balkhan Bay are still topical. Four years later, in 1829, another famous naturalist, A. Humboldt, visited the Caspian Sea area, where he comprehensively surveyed the geomorphologic terraces of the Caspian and observed the fluctuation of the sea level. It should be mentioned that E.F.Kankrin, the Russian Minister of Finance, assisted the expedition. A year later, in 1830, Academician E.Ch.Lenz was the first to set two fixed measuring marks (benchmarks) in Baku and Nargin Island to compare the level values of the Caspian.
Between 1832-1836 G.S. Karelin carried out several surveys along the northeast and east coasts of the Caspian. During these, the biodiversity of Mertviy Kultuk, Kaydak and Kara Bogaz Gol shallow saline bays was comprehensively studied. In 1834, Karelin initiated the building of Novo-Aleksandrovskaya fortress on Kizil-Tash terrace in the Kaydak Bay.
Discussions of the sea level changes began in the early 19th. In 1834 Eichwald first conceived the hypothesis that the periodical rising and lowering of the seabed caused the sea level fluctuation. The sea level was of so much interest, that Baku Customs Department initiated the systematic instrumental observations of it in 1837. For this purpose a special tide-gauge was mounted opposite the customs building.
In 1853-1856 the Caspian commercial research expedition led by K.M.Bair and N.Y.Danilevsky was undertaken, which carried out the most important work on the Caspian ichthyofauna and hydrochemistry. Imperial Zoological Institute officers focused on the fundamental aspects of biodiversity studies and, moreover, worked out a number of sufficient practical recommendations. For instance, they were first to provide scientifically sound instructions for fishing and the methods of the caught fish transportation. The results of these studies were published (Bair, 1953) and the archives are kept in the Russian Academy of Sciences Zoology Institute.
In 1858 the great French writer Alexander Dumas went ashore from Nakhimov’s board in Astrakhan to assess the biodiversity of the Caspian in his own way. Having had an experience of hunting on the Caspian coast, he mentioned this in his geographical novel named From Petersburg to Astrakhan: “While visiting Astrakhan, I had a shooting on the Caspian shore, which abounds in grey geese, ducks, pelicans and seals just like the Seine does in frogs…” (Mauroit, 1957).
In 1860-1862, a famous Russian zoologist Severtsov studied the Caspian part of the Ural River delta resulting in “The Life of Red Fish in the Ural Waters and Its Importance for Ural Fishermen”.
In 1861 Academician Khanykov criticized E.I.Eichwald’s views. The latter believed that the reason of the annual sea level changes is vertical seabed movement, and suggested that they are caused by climate factors. It was highlighted in his article “The Alternating Changes in the Caspian Sea level”.
In 1869 the famous Russian biologist Academician Kovalevsky discovered and described about twenty species of the Caspian invertebrates. In 1876-1877 the Aral Caspian Expedition led by O.A. Grimm was held. This expedition collected extensive data on fauna and flora of these two saline lakes, which was published in several volumes. In 1884 ichthyologist Borodin initiated activity on breeding of Stellate sturgeon at the Ural River. Six years later this author had successful experiments of insemination of sturgeon eggs. With the assistance of Borodin the double fixed net fishing on the Ural River was prohibited and the seine fishery was introduced, which allowed to preserve the young sturgeon individuals.
The Fluctuations of the Caspian Sea Level by N.M. Philippov was published in 1890 criticizing the Eichwald’s and Khanykov views and suggesting the major factor to affect the Caspian Sea level was river input and, primarily, the Volga discharge (Sonne, 2000).
In 1894 a famous Russian geologist N.I.Andrusov and navigator Maximovitch traveled to Kara Bogaz Gol to take samples of local flora and fauna.
In 1897 I.B.Spindler discovered mirabilite layers on the seabed in Kara Bogaz Gol. The same year a famous chemist A.A.Lebedintsev reported on the unique mineral resources available in Kara Bogaz Gol at the 10th International Geological Congress in St.Petersburg.
In 1901 commercial fishing in the open sea of Azerbaijan sector commenced. At the same time the outstanding ichthyologist A.N.Derzhavin began experiments on the sturgeon breeding at Kura river. Later in 1902 unsuccessful attempts to introduce Glossa flounder (Platichthys flesus flesus), mullet (golden grey mullet Lisa auratus è ceaping grey mullet L. saliens), mackerel (Scomber scombrus) were undertaken. In 1904 the Astrakhan Ichthyology Laboratory was established. It contributed a lot into the monitoring of the Caspian fish and served the ground to the ichthyologic database of the Caspian.
In the 1904 a number of scientific and commercial fish surveys led by N.M.Knipovitch were undertaken. The three surveys dated 1904, 1912-1913 and 1914-1915, allowed to identify overall distribution patterns of depths, currents, temperature, salinity, oxygen, hydrogen sulfide, as well as those of plankton, benthos and fish populations. Also, seasonal fluctuations were studied for certain cases. The importance of Knipovitch’s studies cannot be overestimated as they had provided the geographic, hydrologic and hydrobiologic basis for all the subsequent Caspian biodiversity-related studies.
In 1912 the Baku Ichthyology Laboratory was established, which made a major contribution in the Caspian biodiversity studies. Two years later one of the first fish hatcheries was opened at the Kura River.
After the October Revolution, the Committee on Kara Bogaz Gol Bay Biological and Mineral Resources headed by N.S.Kurnakov was formed. In 1919 by Lenin’s direct order the first Soviet natural reserve was established. This was the Astrakhan natural reserve, which made an outstanding contribution to the preservation of biodiversity in the Volga delta and adjacent North Caspian areas. The personnel of the Reserve gathered and processed a high amount of field data during the period of the Reserve’s existence. At present, it has a unique primary database covering almost the whole century.
Notwithstanding the economic difficulties of the early Soviet years, the first volume of The Acta of the Caspian 1914-1915 Expedition prepared by Knipovitch was issued in 1921. The same year Knipovitch published The Caspian Sea and Its Commercial Resources, dedicated to application-specific aspects.
Also, 1921 was the year the first research expedition to Kara Bogaz Gol led by Academicain Kurnakov was undertaken. The expedition purposed to study hydrobiology, hydrology, meteorology and hydrochemistry of the Bay as well as the probability of natrium sulfate deposits here. The findings of the expedition allowed the commercial production of natrium sulfate in the area three years later.
The same year experiments on breeding of a valuable Caspian kutum (êóòóì) species were originated at Kumbashinka River, Azerbaijan, and Samur Fish Hatchery.
The weather in 1925 was extremely cold and frosty when the air temperature reached –250C and many of the bays froze, the Kirov Bay (Kyzykagach Bay now) among them. The high mortality of waterfowl was observed, and the biodiversity of ornithofauna was significantly damaged. The death of a few thousands flamingo was recorded. It was the year when commercial fishing of the Caspian sprat commenced, which had never been netted before. In 1927 Iran Ryba, the first joint Soviet-Iran company, was established, which had worked till 1953 and dealt with fishing along the Iranian part of the Caspian shore.
In 1929 the Volga-Caspian scientific fish hatchery was established on the base of Astrakhan Ichthyology Laboratory. The same year in the Kyzykagach Bay, Azerbaijan, a similarly named reserve was established to provide the protection of wintering waterfowl.
In 1930 the Herring Expedition and 1931-1934 Trans-Caspian Fishery Expedition were held, which considered the commercial fishing in scientific terms. In 1930 the recommenced attempts to introduce the Black Sea grey mullet (Mugil aurarus Risso) and golden mullet (Mugil saliens Risso) were successful. However, the simultaneous activities to introduce the purple gallinule (Mullus barbatus), Black Sea anchovy (Engraulis ensrasicolus) and brill (Psetta maeotica) species to the area proved negative.
In the early 1930s Rhizosolenia plankton algae was accidentally introduced into the Caspian and to 1934-1936 significantly suppressed the local phytoplankton species (see the section about the introduced species). In 1932 the All-Union Scientific Research Institute of Fish Industry and Oceanology began its activities at the Caspian. The work of this Institute can not be overestimated, as its staff initiated a range of studies of the biodiversity, quantitative distribution of aquatic life, bioproductivity phenomena and had developed new adaptation programs. At present, the Institute has one of the most comprehensive bases of primary oceanological and hydrobiological data for the Caspian.
In 1933 the Caspian Sea level decreased from –26.14 m mark. A special session of the SU Academy of Sciences devoted to this event was held. The objective of the session was to discuss the problems of the Volga-Caspian area. The session conceived the idea of moving the input of northern rivers into the Caspian basin with the intended replaced volume determined as 50 km3. Another Caspian natural reserve named Hassankuli Reserve was established in Turkmen SSR the same year to provide regular studies of the wintering waterfowl. It is worth mentioning that the reserve included almost all shallow waters of the Caspian.
The next year, the Special Commission for the Caspian Sea Studies headed by the outstanding Caspian researcher Academician Knipovich was formed under the Soviet Academy of Sciences. The main purposes of the Commission were to coordinate all the Caspian-related studies conducted by the Academy and advise other non-academical institutions on the Caspian-associated issues. As mullet abundance significantly increased for four years after the introduction, the commercial fishing of mullet was first permitted in the Caspian the same year. In 1934 Academician I.G.Alexandrov first suggested isolating Kara Bogaz Gol and Komsomoletz Bays from the Caspian in order to sustain the sea level.
In the middle 1930s a famous scientist Academician L.S.Berg from the Zoology Institution under the Russian Academy of Sciences first proved that the Caspian Sea level had never increased by more than 5 m above the contemporary level, the latter taken –26.26 m as registered in 1925. It is notable that the same the sea level was observed in 2000.
In 1934 a group of Soviet scientists inspired the first research expedition in the Caspian ice conditions.
In 1935 the SU Council of People’s Commissars issued the special decree On the regulation of Fishery and Protection of Fish Stocks, which focused on fishing in the Caspian. It also declared the Soviet section of the Caspian fishery area to be closed for Iranian vessels which usually trespassed the border along Astara – Hassankuli line. A year later the mass commercial fishing with fixed nets was organized in the North Caspian.
In 1937, the State Hydrology Institution led by a famous scientist B.D.Zaykov started studying the Caspian the sea level changes. The Institute contributed significantly in the collecting hydrological data of the Caspian.
In the late 1930s extensive experimental studies of the Azov Sea and the Black Sea fauna naturalization in the Caspian were initiated led by the outstanding hydrobiologist L.A.Zenkevitch, who succeeded to prove that Polychaete Nereis worm and bivalve Abra are an effective food base for sturgeon and other fish, feeding on benthic fauna. Based on these studies 61,000 and 18,000 individuals of Nereis and Abra, correspondingly, were introduced to the Caspian in 1939 and 1940. Both programs of adapting were successful and positively affected the fish industry.
Also, in the late thirties Ichthyology and Hydrobiology Chair of the Leningrad State University headed by Professor Gerbilsky developed a new method of hypothalamic hypophysean injections, which subsequently allowed the mass breeding of the Caspian sturgeons in special fish hatcheries.
In the postwar period, in 1948, the Volga-Caspian Scientific Fishery was reformed into the branch of the All-Union Scientific Institute of Fish Industry providing the extension of staff, improvement of logistics and equipment base, and increase of financing, which significantly assisted the ichthyological researches in the Caspian.
In 1952, the Volga-Don Navigation Canal 101 km long was commissioned. Having nine locks, which elevated the sea level up to 8 m, the canal served as a passage for incidental unplanned introduction of many alien species to the Caspian (see the adequate section). November 11 the same year the strongest storm surge in the Caspian occurred. Strong winds between the Volga and Ural deltas had driven Caspian water over 30 km inland. The sea level rose up to 4.5 m resulting in loss of people and animals. The catastrophic surge demonstrated possible destructive effects of the strong winds on the onshore biodiversity. In 1954 Ust-Kura Strugeon Hatchery in Azerbaijan was commissioned. This hatchery, along with many others, utilized the method of hypothalamic hypophysean injections discovered by professor Gerbilsky in Leningrad before the War. A year later Kizan Fish Hatchery located in the Volga delta was commissioned. Five years later this hatchery put into operation the inconnu fish hatchery. In 1962 the activities for chum salmon (Oncorhynchus keta) adapting in the Caspian were initiated. In 1965 first studies of commercial sterlet breeding were originated in the Volga delta area.
The post-war period Soviet ichthyologists did a lot for the preservation of the Caspian fish biodiversity with the main focus on sturgeon. Due to their efforts and the operation of fish hatcheries valuable fish species were preserved. However, illegal and uncontrolled sturgeon fishing in the Caspian often brought these efforts to nothing. Therefore, after the Trans-Caspian Sturgeon Survey in 1962-1963 the Soviet Government put a complete blackout on sturgeons fishing in the open sea. It is the scientists’ belief that due to this blackout the existing stock of sturgeons has been preserved.
Besides the prohibitory measures, the Soviet Government decided the establishment of many new scientific institutions in the middle 1960s. The activity of these institutions was to ensure the preservation of the Caspian communities' biodiversity. Thus, the Caspian Scientific Research Institute of Fish Industry was established in 1965. This Institute played an important part in the Caspian biological resources studies, is still staying in the lead of the ichthyological and hydrobiological studies in the region, and continuing the Caspian biological resources monitoring and having developed a valuable database.
In 1968 the Caspian Ornithological Station and the Institution of Water Issues to the SU Academy of Sciences were established using the Astrakhan Reserve base.
The same year “The Atlas of the Caspian Invertebrates” was issued, which became a valuable manual for biodiversity studies.
However, along with the Caspian biodiversity maintenance measures, Soviet Government activities in sixties’ included secret military trials as well.
As per the decree of the government, the first trials of giant ekranoplans were conducted in the vicinity of Chechen Island, which also served a secret polygon and laboratory base for design and testing of these military carriers. Their high-speed flights close to the water surface caused the loss and damage to many waterfowl, seal and fish individuals. The animals were killed or hurted by the overpressure formed under the bottom part of flying ekranoplans. Also, missiles launched from military polygons and vessels affected the Caspian biodiversity adversely. The adverse effect was caused by biocidal propellants, such as hepthyl, which led to the total intoxication of the water and land living matter.
Unfortunately, the scale of these effects is still assessed quite approximately, since all the military missile accidents were kept top secret thereat (Zaymayev, 2001)
At present Caspian countries have a wide range of data collected during a long period of time. The data was received by various organization during single or routine observations. Data comprehensiveness differs with areas, years, seasons, accuracy. Consider the data by components.
The Caspian hydrobiology has been constantly studied from the time of the 1st Trans-Caspian Expedition in 1934. During the postwar period, the Caspian Scientific Research Institute of Fish Industry held regular annual seasonal observations, which soon covered the entire area of the Caspian except Iran waters.
Zoology and Botany Institutes to the Soviet Academy of Sciences and other scientific institutions of the USSR conducted regular expeditions in the area. Numerous monographs and reviews dedicated to the species diversity, distribution patterns, abundance, and biomass of phytoplankton, zooplankton and benthos in the Caspian were published (Proshkina-Lavrenko and Makarenko, 1968; “The Atlas of the Caspian Invertebrates”, 1968; Birstein et al., 1968; “The Caspian Sea…”, 1985; “The Caspian Sea…”, 1996; etc.)
In the nineties, the Caspian Sea studies were drastically reduced, since the post-Soviet Caspian states were unable to sustain the existing level and volume of studies. Therefore, the most complete and systematic data refer to 50s-80s. The primary data are scattered, being held by unrelated institutions previously involved, which makes the creation of an integral database difficult. It should be noted, that the most part of studies tended to deal with quantitative changes in fish stocks, that is predominant species, rather than with biodiversity identification or verification.
Similarly, fish species diversity studies, commenced in 19, allowed the accumulation of long-term data of the Caspian fish biology, abundance, distribution and harvesting dynamics. Primarily, the data refer to commercial fish species leaving non-commercial one out of the research scope. The outcome of this is that the Caspian fish species are described per 1968 report (Kazancheev, 1968) that does not include the records of changes, which occurred with time.
In contrast, the information on the Iran section of the Caspian is poorer. Regrettably, “The National Report on Biodiversity in the Iran Section of the Caspian” does not provide sufficient data on long-term observations, area, source and accessibility of primary data. The reports on the Caspian shoreline are limited to those developed for some areas. Thus, Zaberzhinskaya and other authors deliberately described the higher water flora of Azerbaijan coastline (Zaberzhinskaya et al., 1967; Zaberzhinskaya, 1968; Petrov, 1967). In the subsequent years other authors expanded and updated the existent materials. The North East Caspian areas were to various extents studied within The Environmental Assessment by OKIOC in 1994-2000.
A number of monographs, including “The Birds of the USSR” (1987, 1988), “The Birds of Kazakhstan (1960, 1962,1970, 1972, 1974), “The Catalogue of Azerbaijan Birds (D.G.Tuayev, Baku, 1996) and numerous articles related to certain areas ornithofauna contain more extensive data on bird biodiversity.
Particularly, the staff of reserves located in the areas where the most diversity and abundance of birds is observed had collected wide-ranged data. Among them, there are the Volga estuary (Astrakhan biosphere State Reserve), Kizlar Bay (Daghestan State Reserve), Agrakhan Peninsula and Bay (Agrakhan Reserve), Gyzylagach Bay (Gyzylagach Reserve), Turkmenbashi Bay (Khazar State Reserve), Southern Coast of the Caspian (the complex of Iran reserves). Local observations conducted by shooting preserves staff and ornithologists from the national Academic Zoology institutes keep field records of bird populations and classify the data. The materials include data on species composition of resident, migrating and wintering birds, their seasonal distribution patterns, major habitat and species status.
The coastal vegetation significantly varies from area to area subject to climate, relief and soil properties. In this relation the available descriptions refer to various unrelated administrative and floristic areas. Azerbaijan coastline vegetation has been featured in the paperwork by Hajiyev (1976, 1992).
Similar topics for Kazakhstan coastline are reported on by Kurochkina et al. (1992-1995) and Safronova (1996). Territorial coverage as well as the timing of observations vary. Study materials are mainly accumulated in the national botany, ecology and agriculture institutions.
Coastline fauna was described in fragments. Along with topic related paperwork, summary monographs for some regions exist (Alikperov, 1978; “The Animals of Azerbaijan, 2000; Kasymov, 1987, 1994; Borkin, Darevsky, 1987; Paraskiv, 1956; The Mammals of Kazakhstan, 1969-1983, etc.)
The data on the Caspian insects is limited in range. For Kazakhstan section it comes to 50s-70s and covers only 30-35% of the species existent. The similar situation is found for other regions.
Information of amphibians appear the most complete and reliable. Along with that, Amphibian species are quite poor in diversity. Information on reptile and mammals is wide-ranged in timing and reflects their main qualitative features. Material on the Caspian fauna are concentrated mainly in zoology and ecology Institutions, reserves and game-preserves.
The available information allow qualitative description of the biodiversity of the Caspian and identification of encountered problems. But, it is still insufficient for quantitative substantiation of anthropogenic impact on the species diversity.
Notwithstanding the information abundance, the correct, properly sourced analysis of biodiversity is quite complicated. The accessibility of all the existent data being unassured and direct links between specific table and evaluations and databases lacking, this review cannot be considered as providing full and reliable analysis of available knowledge. In these terms, the lack of sorted information at the lower management levels is the main problem, which may bring competitive results once solved.
The insufficient system of community, landscape and habitat identification and the deficiency of accurate evaluation criteria of their importance for biodiversity preservation, similar to those accepted in Europe within Natura 2000 program, are the main causes that complicate the assessment of existing biodiversity. Still, the main problem is the deficiency of the proper base such as species distribution maps with a database, habitat and community maps and other. The lack of this basic documentation reduces the reliability and value of the information collected.
Information problems encountered in developing such documentation are the key issues of managing the human-to-environment relations. Any activity in this field should be based on real and not fictitious data and subsequent relations, which should be completely accessible and verifiable.
An important issue that complicates conclusions on biodiversity status and trends of its alteration is the absence of over-year monitoring. More or less systematic studies of the Caspian and the shoreline in the 50s-80s were reduced to the minimum later in the 90s.
Some gaps existed in previous years as well, leading to the lack of accurate data on biodiversity and its changing for any of the organism groups of the Caspian.
In 90s the hydrobiological activities in the east section of Central and Southern Caspian nearly stopped. In a limited range they were conducted in the west section and North East Caspian. In the 90s The sea level rising and significant water desalination were observed in the North Caspian, which influenced the adjacent section of the Sea. This definitely assisted the partial replacement of the sea-water organisms with brackishwater and freshwater ones. However, it was not sufficiently reflected in studies due to deficient observations. Also, recent studies contain insufficient data on seasonal changes in phytoplankton and zooplankton species diversity.
Non-commercial species of the Caspian fish being left out of due observations, the number of determined fish species range from 124 to 156 per various material sources and the exact quantity of the species available is unknown. For some species, genetic studies are required to define distinctions between them. The Red Books of the Caspian states register only commercially valuable fish species. “The Fish of the Caspian Sea” by Kazancheev, published in 1968, is still the main source of the Caspian fish biodiversity information.
Information on observations in Iran section of the Caspian is very poor, since the studies are likely to be limited to those conducted by the Caspian Scientific Institution of Fish Industry in 1995-1997.
Although abundant, the Caspian coastline vegetation lies mainly out of the sea-affected scope as its biodiversity is not determined with the close presence of the sea. Specific areas where sea influence is obvious either have not been studied or were not considered to be a specific botanic province. The existence of the most of the species, previously classified as rare, endemic, extinguishing or endangered, require field verification.
Extensive data is available for the majority of species, but due to variety of causes they may not be utilized to assess the effect of a specific industrial facility on the wildlife. The main reasons for this are as follows:
All the above prove the necessity in adapting the existing diverse data and bring the characteristics (even along with quantitative values) to parameters suitable for impact evaluation and expression in economic terms.
This data deficiency review concludes in the necessity to extend the range of field activities and develop the system of constant integral program-based environmental monitoring covering the entire Caspian area. The monitoring should be complex and should include space, air and land observations.
Simultaneously, the development of multilevel database is required, which would contain long-term diverse information for each biotic type and which would make accessible the materials resulting from numerous scientific, design, monitoring and administrative institutions over-year activity.
An apparent need in the development of databases for each country and the Caspian Sea exists, which requires provision of uniform information for data collection and comparison, those including:
Unification of data collection methods used by individual institutions, non-governmental organizations and research institutes of the Caspian states is desirable. Along with the data collection and study methodology, recording, analysis and reporting methods should be standardized. It would allow comparing the data throughout the region assuming their uniform collection, processing and representation (Monitoring Methodology, 2000).
Analysis of data collection, processing and representation systems throughout the Caspian region is recommended to promote the overall standardization. The review should contain, among others, the terminology and software common for the accepted Geographical Information Systems (GIS).
Within biodiversity monitoring a wide range of studies may be conducted. The Caspian Sea should be considered as an integral extensive ecosystem with stable and variable species of interest. The territorial water of the states can be studied using traditional observation methodology, with mobile species requiring more extensive methods. This is necessary because of the seasonal migration of many sea species. Thus, some waters of the North Caspian may be important as the locations of reproduction and pasturing of fish, but these species may winter in the South Caspian as well. Due to this phenomenon, close connections between the countries of the region while planning and conducting sea studies are important.
Similarly, wetlands may be studied up to the habitat and specific composition management within a separate country. However, many of the wetlands of the Caspian are important for migrating and wintering birds, some of them ranged valuable within pan-European and regional scale. Therefore, some coordination in the fields of study methodology and importance of biodiversity preservation should be provided. Beside these practical considerations, an agreement on the direction and intensity of biodiversity studies should be reached based on cooperation and interaction principles.
Environmental observations being the major part of the sea, water and wetlands ecosystem studies, the unification of research methodology to allow data uniformity is of great significance.
The preservation of sea habitats is immediately associated to the implementation of Bucharest Convention of April 21, 1992, signed by all the Caspian states. This convention incorporates the Protocol on the Protection of Sea Habitats from Shore- Originated Pollution. This Protocol has two Appendices, which correspondingly detail dangerous and toxic materials management.
A number of environment and nature preservation initiatives and international agreements such as Bern Convention, Biological Diversity Convention and Ramsar Convention participated and signed by all the Caspian states. They include specific environmental agreements on such issues as protection of migrating birds and strategically valuable wetlands. Besides, within national legislation, some countries individually gave certain areas and habitats the status of national parks and reserves. Hence, a similar approach to the understanding and protection of the key habitats may be stated.
Among the coastline conserved areas are such as the Dunai Estuary and Biosphere Reserve, some areas of Colchis and natural reserves.
Certain plans of area management are developed and implemented in the coastline countries, in which different methods of administration are likely to be used. Also, the coastline countries are preparing a number of future initiatives like joint coastal zone administration, which include biodiversity as a direction of activity. Such initiatives provide a ground for the further consideration of biodiversity, ideally on the structured and agreed basis.
It is accepted that during the recent decades the biodiversity was adversely affected by the following anthropological factors:
Also, the low quality of water affects them as well as the river ecosystem. There is an obvious necessity in integration of the economic development policy, agriculture and power industry with the policy related to the stability of the environment and biodiversity in order to assure the compliance with the development policy and purposes of The Biodiversity Strategy of the Caspian.
Besides, it is possible to establish a regional program like MedWet in relation to the Caspian Sea. Under the Ramsar Convention on wetlands and with the support from the European Commission, MedWet was conceived in the early 90s as an experiment in the field of long-term cooperation on governmental, non-governmental and personal levels and as a joint initiative being part of efforts for preservation and reasonable utilization of the Mediterranean Sea. MedWet provides the scope for wetland site management mainly focused on:
MedWet includes the data study and evaluation methodology successfully tested in non-Mediterranean countries which in major may be transferred into the Caspian context.
Another way to preserve the sea environment is the establishment of reserve and national park network in the key habitat locations of the Caspian. International reservation areas in conformance with the transboundary distribution and migration patterns of species are particularly effective. More profound arrangements and closer cooperation at the levels ranging from local and national to regional and global are required to preserve species and ecosystems that transgress human made boundaries. The institutions at all the mentioned levels are to take key and auxiliary parts in the sea environment preservation.
The Caspian reserve network requires extension with regard to the most important reproduction, feeding and wintering sites and migration routes of fish and sea mammals.
The development, implementation and completion of plans for extinguishing species is another primary task of biodiversity preservation strategies. The necessity of developing the existing plans as well as other instruments, such as recommendations following the conventions that may be considered minor action plans, was emphasized at the Pan-European Biology and Landscape Strategy, the first session of which was held September 12-13, 2000 in Strasbourg (Council of Europe, UNEP). It was recognized that planning methodology may be diverse and many models may be suggested.
Some extinguishing plants and animals, such as pelicans, may serve as leading species for campaign drives.
The notion of a leading species includes the selection of clearly identified species, which may be "observed" by the community, and the species indicating the quality of the environment. The local community may be involved in the collection of data on habitats and biodiversity, which task otherwise would require considerable efforts.
"Knowledge should lead the action; the intellectual understanding is useless if not associated with moral imperative". (Berry, "The Preservation and Development for the Great Britain. The Response to the World Preservation Strategy." Kogan Page London, 1983).
(Anon, 1994) The UK Action plan
Bratanova O. N., Svitoch A.A. Palaeosalinity of the Pleistocene Caspian Sea according to Ostracods // The First International Conference “Application of International Micropalaeontology in Environ-mental Sciences”. Porter Super-Center for Ecological and Environmental Studies and Institute for Nature Conservation Re-search, Tel-Aviv University. Tel-Aviv — Israel, June 15–20, 1997. P. 42–43.
Budyko M.I., P.Ya. Groisman, 1991. Climate of the USSR in 2000. Soviet meteorology and Hydrology, No. 4, p. 58–66.
Clegg J.S., Jackson S.A. Significance of cyst fragments of Artemia sp. recorded from a 27,000 year old core taken under the Great Salt Lake, Utah, USA. Int. J. Salt Lake Res., 1997, vol. 6, No. 3, p. 207–216.
Dawson, A.G., 1992. Ice Age Earth: Late Quternary Geology and Climate. London, U.K.: Routlege.
Degens E.T., Paluska A. Tectonic and climatic pulses recorded in Quaternary sediments of the Caspian–Black Sea region // Sedimentary Geology, 1979, 23. Pp. 149-163.
Dumont H., 1995. Ecocide in the Caspian Sea. Nature, vol. 337. Pp. 673–674.
Dumont H., J., 1998. The Caspian Lake: History, biota, structure, and function. Limnol. Oceanogr., 43(1). Pp. 44–52.
Dumont H., J., 2000. Endemism in the Ponto-Caspian fauna, with Special Emphasis on the Onychopoda (Crustatcea). Advances in Ecological Research, vol. 31. Pp. 181–196.
Ekman S., 1916. Systematische und tiergeographsche Bemerkungen uber einiger glacialmarine Relikte des Kaspischem.
Georgievsky V.Yu., Shiklomanov J.A., 1994. Impact of Economic Activity and Global Changes of Climate on Runoff in the Volga Basin. In: Study Report: Climate Variability and Change in the Commonwealth of Independent States (CIS): Forecasting Climate – Related Impacts and Societal Responces to them. Ed. by I.S. Sonne, UNEP. Pp 209–246.
Golubev G.N., 1997. Closed areas and the Case of the Caspian Sea Basin. In: Publication of Japanese-German Center, Berlin. Vol. 9. Conference: Perspectives of Eurasia as a Field of Global Communication. 05-07.07.92, pp. 125–136.
Grosswald M.G., 1980. Late Weichselian Ice Sheet of Northern Eurasia. Quaternary Research, 13, p. 1–32.
Hansen J., I. Fung, A. Lacis, D. Rind, S. Lebedeff, R. Ruedy, G. Russell, 1988. Global climate change as forecast by Goddard Institute for Space Studies three-dimensional model. Journal of Geophysical Research, 93, p. 9341–9364.
Ivanov V.P., Kamakin A.M., Ushivtzev V.B., Shiganiva T., Zhukova O., Aladin N., S.I. Wilson., Harbison R.G., Dumont H., 2000. Invasion of the Caspian Sea by the comb jellyfish Mnemiopsis leidyi (Ctenophora). Biological Invasions, 2. Pp. 255–258.
Jones R.W., Simmons M.D. A review of the stratigraphy of Eastern Parathethys (Oligocene–Holocene) // Bull. nat. Hist. Mus. Lond. (Geol.), 1996, 52(1). Pp. 25-49.
Kellogg W.W., 1991. Response to skeptics of global warming. Bulletin of the American Meteorological Society, 74, p. 241–253.
Kerr R.A., 1989. Hansen vs. The world on the greenhouse threat. Science, 244, p. 1041–1043.
Klige R.G., M.S. Myagkov, 1992. Changes in the water regime of the Caspian Sea. Geojournal, 27, p. 299–307.
Kosarev A.N., Yablonskaya E.A. 1994. The Caspian Sea. SPB. The Hague, 259 pp.
Krylov V.I. Ecology of the Caspian seal. Finnsih Game Res., 1990, 47: 32–36.
Lamb H.H., 1977. Climate: Present, Past and Future, vol. 2, London, U.K.: Methuen.
Lee R.F., 2001. New data on cysts from salt lakes. Int. J. of Exobiol., vol. 4, No. 2, p 68–76.
Letolle R., Mainguet M., 1996. Der Aral See: eine okologishe katastrophe, Springer Verlag, Heidelberg, 517 p.
Mordukhai-Boltovskoy P.D., 1979. Composition and distribution of Caspian fauna in the light of modern data. Int. Rev. Gesamten Hydrobiol. 64. Pp. 383–392/
Ozturk et al., 1999. NATO/Committee on Challenges of Modern Society. NATO/CCMS Pilot Project. Review of Environmental Projects of the Caspian Sea for the Planning of Future Activities. November 1999. Report No. 239.
Rodionov S. N. Global and regional climate interaction: the Caspian Sea experience. Water Science and Tchnology Library, vol. 11. 1994. 241 p.
Rogl F., 1998. Palaeogeographic Considerations for Mediterranean and Paratethys Saeways (Oligocene to Miocene). Ann. Naturhist. Mus. Wien, 99. Pp. 279–310.
Sars G.O. 1927. Notes on the Crustacean fauna of the Caspian Sea. In: A Tribute to Prof. N. M. Knipovich. M.: VNIRO. (in Russian)
Schneider S.H., 1990. The global warming debate heats up: an analysis and perspective. Bulletin of the American Meteorological Society, 71, p. 1292–1303.
Svitoch A.A., Yanko V., Bratanova O. N., Palaeosalinity of Intercontinental Basins by Microfaunistic Data (the Caspian Sea during the Pleistocene) // The First International Conference “Application of International Micropalaeontology in Environmental Sciences”. Porter Super-Center for Ecological and Environmental Studies and Institute for Nature Conservation Research, Tel-Aviv University. Tel-Aviv — Israel, June 15–20, 1997. P. 112–113.
Agamaliev F.G. The Infusoria of the Caspian. Systematization, ecology and zoogeography. L.: Nauka, 1983, P. 252.
Aladin N.B. The Osmoregulation Peculiarities of the Cyprideis torosa crustacean (ðàêóøêîâîãî ðàêîîáðàçíîãî) from the Seas of the USSR", // Zoological Journal, 1989, 68, 7. P. 40-50.
Aladin N.B., Plotnikov I.S., 1995. The Changes of the Aral Sea Level: paleolimnological and archaelogical evidences. The Papers of Zoology Institute under the Russian Academy of Sciences, V. 262, p. 17–36.
Aladin N.B., Plotnikov I.S., 2000. The Danger of Large-scale Ecological Catastrophy in the Caspian (The Comparative Analysis of Causes and Effects of Ecological Crises in the Aral and Caspian), The Caspian Bulletin, Issue 4. P. 112–126.
Alikperov A.M. The Amphibia and Reptiles of Azerbaijan. Baku, Elm, 1978, p. 263.
Atamuradov Kh.I., 1999. The Biological Resources of Gara Bogaz Gol Bay. Turkmen Academy of Sciences Publishing House. P. 48.
The Atlas of the Caspian Invertebrates. M.: Food Industry, 1968. P. 415.
The Atlas of the Kazakh SSR, v. 2, 1, Almaty, 1982, p. 81.
Bagirov R.M. The Azov and Black Sea Species Introduced to the Caspian benthos and biofouling. Abstract from the Doctoral Thesis, Baku, 1989.
Badamshin B.I. The Bioilogical and Economical Grounds for Transformation of the Caspian Seal Harvesting into Rational Industry. // The Third All-Union Workshop of Sea Mammals. M-L., 1966.
Badamshin B.I. The Abundance and Commercial Stocks of the Caspian Seals. // The Sea Mammals, Ì., 1969.
Belik V.P. The Peculiarities of Fall Migration of Geese in the North Caspian. // Brand Goose. 2. 1996. ñ. 253-257.
Belayeva V.N., Ivanov V.P., Zailanova V.K. The Scientific Grounds for Stable Fishing and Regional Distribution of Commercial Sites of the Caspian. Ì., 1998.
Berg, 1928. About the Origins of the Northern Elements in the Caspian Fauna. The Records of USSR AS, 14, 3.
Birstein et al. The Atlas of Inverebrates of the Caspian. L., Nauka, 1968, p. 415.
Birstein, 1939. The North Caspian Benthos. Zoology Journal, 18, 3.
Bokova E.B. in The Biological Resources of the Caspian collection. Astrakhan, 1992.
Borkin L.Y., Darevsky I.S,. The Listing of Amphibians and Reptiles of USSR. The Amphibia and Reptiles of the reserve areas. The collection of research papers of CNIL Glavokhota, Russian Federation, Ì., 1987. p. 128-160.
Bortnik V.N., Kuksa V.I., Saltankin B.P., 1997. The Existing geological and ecological standing of the Volga-Caspian basin. Water Resource, V. 24, issue 5, p. 548–555.
Budyko M.I. The Evolution of the Biosphere. L.: Hydrometeoizdat, 1984.
Budyko M.I., Yefimova N.A., Lobanov V.V, , 1988. The Level of the Caspian in Future. Meteorology and hydrology, 5. P. 86–94.
The Revivial of the Volga – a Step to the Salvation of Russian., Ì.- N.Novgorod: Ecology, 1996.
Voynova I.A, , Alikperov V. The Biological Resources of the Caspian – the collection. Astrakhan, 1992.
Hajiev V.D. The Vegetation of Azerbaijan. Baku, Ishig, 1976.
Hajiev V.D. The Vegetation of Azerbaijan. The Vegetation Map of Azerbaijan. Scale 1:600 000, 1992.
Hidayatov Y.Kh. The Fox as a Effective Animal. Elm ve Hayat. Issue 8, 1967.
Hydrometeorology…, 1996
Glukhovtsev I,. The Kazakhstan Section of the Caspian. Caspian Magazine. 1997.
Gorbunov G.S. The Assessment of Physiological Condition of Some Fish Species of the Caspian under the Effect of Stratal Water. Astrakhan, 1989, p. 414.
Ctenophora Mnemiopsis leidyi (A. Agassiz) in the Azov and Black Sea: the Biology and Effects of Introduction. Edited by Biology Doctor, Professor S.V.Volovik. Rostov-on- Don: BKI, 2000. P. 500.
Humboldt A. 1915. The Central Asia.
Derzhavin A.N., 1912. The Caspian Elements in the Volga Basin Fauna. Ichthyology Labiratory. 2, 5.
Dolgushin I.A. et al. The Birds of Kazakhstan, in 5 volumes, Almaty, v. 1, 1960, v. 2, 1962, v. 3, 1970, v. 4, 1972, v. 5, 1974.
The Wildlife of Azerbaijan. V. 3. Baku, 2000.
Zaberzhinskaya E.B. The Flora of Macrophyte Algae of the Caspian. The Abstract of thesis for Biology Doctor. Baku, 1968, p.16.
Zaberzhinskaya E.B., Zinova A.D., Petrov K.M., The Macrophytes of the Caspian at the Azerbaijan Coastline (Conferva, Phaeophyta and Rhodophyta). The Experience of Geomorphological and Hydrobiological Studied çåëåíûå, áóðûå è êðàñíûå âîäîðîñëè). L., Nauka, 1967, p. 158–203.
Zaykov, 1946
Zenkevich L.A. The Fauna and Bioproductivity of Sea. M., Sovetskaya nauka, 1947.
Zenkevich L.A., 1963.The Biology of the USSR Sea. Ì.: Nauka.
Sonne I.S., 2000. Three centuries at the Caspian (The Synchronism of Major Historical Events of XVIII–20 cc.). Ì., 72 ñ.
Ivanov V.P. The Biological Resources of the Caspian. Astrakhan, The Publishing House of Caspian SIFI, 2000. P. 100.
Ivanov V.P., Sokolskiy A.F., The Scientific Basis for the Strategy of Protection the Caspian Biological Resources from Oil Pollution. Astrakhan: The Publishing House of Caspian SIFI, 2000. P. 181.
Isuyev A.R., Gabibov M.M. The Influence of Drilling Muds on the Kutum at the Early Stages of Development. Astrakhan, 1989, p. 423.
Kazancheev E.N., The Fish of the Caspian Sea. Ì., 1981.
Karpevich A.F., 1975. Theory and Practice of Water Organism Acclimatization. Ì.: Food Industry, p. 432.
The Caspian Sea. The Hydrochemical and Oceanological Grounds for the Bioproductivity Formation. The Marine Hydrometeorology and Hydrochemistry. V. 6, Issue 2. Hydrometeoizdat, 1996, p. 260.
The Caspian Sea. The Fauna and Bioproductivity. Ì., Nauka, 1985, P. 276.
Kasimov A.G. The Wildlife of the Caspian Sea. Baku, Elm, 1987. P. 156.
Kasimov A.G. The Qualifier of Copepoda Crustacenas of the Caucasus. Baku, Elm., 1997. 196 ñ.
Kasimov A.G. The Ecology of the Caspian Lake. Baku. Azerbaijan, 1994. P. 240.
Kvasov D.D., Mamedov E.D., The Development of Irrigation and its Effects on the Water Balance. // The History of the Lakes of Sevan, Issyk-Kule, Balkhash, Zaysan and Aral Èñòîðèÿ îçåð Ñåâàí, Èññûê-Êóëü, Áàëõàø, Çàéñàí è Àðàë. — L., 1991. P. 227–230.
Knipovich N.M. 1938. Hydrology of seas and brackish waters. M.-L.: “Pischepromizdat”. (in Russian)
Kosarev A.N., Tuzhilkin V.S., 1995. The Climatic Thermohaline Fields of the Caspian. Ì., P. 91.
The Red Book of the Azerbaijan SSR. Baku, 1989.
The Red Book of Kazakhstan. V.1. Animals. Part 1. Vertebrates. Almaty, 1996, p. 350.
The Red Book of Kazakhstan. V.2, Almaty,1981, p.260.
The Red Book of the Russian Federation. Animals. M., Rosselkhozizdat, 1983, p.453.
The Red Book of the USSR. Vol. 1 and 2. M, 1984.
The Red Book of Turkmenistan. Vol. 1 and 2. Ashgabat, 1999.
Krylov V.I. The Findings of the Caspian Seal Studies in the Ural delta for 1978-1984 //Marine Animals. Ì., 1986. P.1-69.
Krylov V.I. The Abyndance of the Caspian Seal in the Eastern "Shalygs" of the North Caspian.// The Study, Protection and Rational Utilization of Marine Mammals. Astrakhan, 1982. P.184-185.
Kuznetsov V.I., Martynova E.F. The Listing of Lepidoptera of the Middle Ural. The Acta of Zoology Institute of USSR AS. Vol. 16, 1954.
Kurochkina L.Ya. The Deserts of Kazakhstan and the Extent of Their Assimilation. // The Rare Animals of the Deserts. Almaty, 1990, p. 7-23.
Kurochkina L.Ya. et al. The Reports of the Field Studies in 1992-1995 and 1999. (the forms of descriptions, maps of vegetation, ecosystems and desertification)
Kurochkina L.Ya., Mirzadinov R.A., The Ecothones of Deserts and Their Classification. //The Problems of Desert Assimilatioò, 1985, No2, p. 34-40.
Cousteau F., 2000. The Caspian Sea. The Report of the Oceanic Expedition of Cousteau Society under the aegis of UNESCO/MOC, May-August 1998. The Caspian Bulletin. Issue 4. P. 84–99.
Levshakova V.D. Certain Ecological Features of the North Caspian Phythoplankton. // The Acta of Caspian SIFI-1971.-Vol.26. – P.67-82.
Mayev E.G., Maeva S.A., Nikolaev S.D. New Data on the Holocene History of the Aral Sea. // The Paleography of the Caspian and Aral Seas in Cainozoic Era. Part II. — Ì., 1983. P. 133–144.
Makrushin A.B, 1985. The Anhydrobiosis of the Early Water Vertebrates: the preservation of vital capacity in dried state. Ì.: Nauka, P. 104.
Monitoring Methods in the Caspian Sea. Edited by A. Kasimov, Baku. Áàêó: «GAPP-POLIGRAF», 2000. P. 58.
The Animals of Kazakhstan. Almaty: vol. I, part I, 1969; vol.. I, part 2, 1977; vol.. I, part 3, 1978; vol. 2, 1980; vol. 3, part I, 1981; vol. 3, part 2, 1982; vol. 3, part 3, 1983; vol. 3, part 4, 1984; vol. 4, 1985.
Mordukhai-Boltovskoy F.D., 1960. The Caspian Fauna in the Azov and Black Sea Basins. Ì: USSR Academy of Scienced Publishing House.
Morozov B.B. The Wintering Sites of Minor Swans. // Brand Goose. Êàçàðêà. 2. 1996. P. 237-243.
Mauroit A., 1957. The Three Dumas. The Outstanding Lives Series. Ì.: Molodaya Gvardia.
The National Strategy and Action Plan for Preservation and Balanced Utilization of the Biodiversity. Kokshetau, 1999, p. 356.
Nevesskaya L.A., Goncharova I.A., Ilyina L.B. et al. The History of ParaThetis Neogene Molluscs. Ì.: Nauka, 1986. P. 208. (The Acta of Paleonthology Institute of USSR AS; vol. 220)
Kes A.S. 1960, The Lower Course of the Amu-Dariya; Sarykamysh, Uzboy. (The History of Formation and Colonization // The Materials of Khoresm Expedition - Ì., 1960. Issue 3. P. 348.
Novikov G.A. The Predators of USSR Fauna. The UUSR Academy of Sciences Publishing House. M-L., 1965.
Paraskiv K.P. The Reptiles of Kazakhstan. Almaty, 1956. P. 229.
Pakhukskiy A.I. The Piscivorous Birds of USSR Southern Seas and Their Adverse Effects. Ì., MOIP, 1951.
Petrov K.M. The Underwater Vegetation of Azerbaijan Coastline. The Experience of Geomorphological and Hydrobiological Studies of the Coastal Zone. L., Nauka, 1967, P. 103–157.
Proshkina-Lavrenko A.I. The Plankton Algae of the Caspian Sea. L., Íàóêà, 1968, P. 291.
The Birds of Kazakhstan, in 5 volumes. Almaty: 1960, 1962, 1970, 1972, 1974.
The Birds of the USSR. Ò. 4, L., Íàóêà, 1952, P. 636.
The Birds of the USSR. Galliformes. Gruiformes. Ì., Nauka, 1987. P.528.
The Birds of the USSR. Laridae. Ì.: Nauka. 1988. P.416.
Romanova N.N., 1958 The Distribution and Ecological Features of North Caspian Amphipoda è Cumacea. The Reports of USSR AS. Vol. 121, Issue 3, P. 553–556.
Romanova N.N. The Survival of Some Amphipoda of the North Caspian at the Various Levels of Salinity The Acta of VNIRO. Vol. 38, issue 1, 1959, P. 277-296.
Russian on the Way to the Stable Development, ÐÝÔÈÀ, Ì., 1996.
Rubanov I.B., Ishniyazov D.P., Baskakova M.A, Chistakov P.A. The Geology of the Aral Sea — Tashkent, 1987. P.248.
Salmanov M.A. The Role of Microflora and Phytoplankton in the Production Processes of the Caspian. Ì.: Nauka, 1987. P.214.
Safronova I.N. The Deserts of Mangyshlak. ÑÏá., 1996, P.210.
Starobogatov Ya.I. The Systematization and Paleonthology Dreissena polymorpha (Pall) (Bivalvia, Dreissenidae) 1994. P. 18-46.
Starobogatov Ya.I. The Mollusk Fauna and Zoogeographic Zoning of Continental Water Bodies of the Earth. L.: Nauka, 1970. P.372.
Taktakishvili I.G. About the Pliocene History of Molusk Fauna of ParaThetis. Tbilisi: Mecniereba, 1977. P. 135.
Tuaev D.G. The Cataloque of Azerbaijan Birds. Baku, 1996.
The Fauna of the USSR. Birds. Charadriiformes. Sandpipers. Vol. 2, Issue 1, part 2. Ì.-L., Nauka, 1961, P. 500.
The Fauna of the USSR. Birds. Charadriiformes. Sandpipers. Vol. 2, Issue 1, part 2. Ì.-L., Nauka, 1962, P. 432.
Fedorov P.V. About Some Issues of the Caspian and Aral Paleogeography in the Late Pliocene and Pleistpcene. // The Paleogeography of the Caspian and Aral Seas in the Cainosoic Era. Part. I. — Ì., 1983. P.16.
Fedorov P.V. About Some Issues of Holocene History of the Caspian and Aral. // The Changes of Wet Aral-Caspian Region in Holocene.— Ì., 1980. P. 19–22.
Fedorov P.V. The Stratigraphy of Quarternary Deposits in the West Turkmenia and Their Position in the Integral Stratigraphic Scel of the Caspian Region. // The Acta of the Geology Institute of the USSR AS — Ì., 1957. Issue 10. P. 300.
Fedorovich B.A. The Ancient Rivers of Turan Deserts // The Materials on the Quarternary Period for the USSR. Vol. 3. — Ì., 1952. P. 93–100.
Chesunov, 1978. New Species of Free Nemathoda of the Caspian. Zoology journal, 57, 4. P. 505-511.
Chuykov Y.S., 1994. The Zooplankton of the North fore-Caspian and the North Caspian. The Astrakhan Committee on Ecology and Natural Resources.
Eberzin A.G. About the Origin of Pliocene Genera of Kardiida in Euxinian Basin. The Acta of Paleonthology Institute of the USSR AS. 1949. Vol. 20. P. 209-232.
Abrasion (Lat. abrasio – erasure) - the process of wave and water induced destruction of sea and lake shores.
Abyssal (Gr. abyssos – bottomless) - the part of seabed with a relatively weak water motion, constant temperature, lack of light and poor fauna.
Adiabatic process – a thermodynamic process when a system makes no heat exchange with the environment.
Allochthons – species (genera, classes of organisms) that entered the given area in the result of migration from the location they emerged in course of the evolution.
Anoxia – in this case, lack of water-dissolved oxygen.
Area (Lat. area – space, room) - the part of land surface within which a specie, genus, class of animal or plant is found.
Arid climate – dry climate with high air temperature and low precipitation typical for deserts and semideserts..
Autochthon – (Gr. autochthon - local, aborigine) – is used to name the components of aborigine fauna and flora.
Benthos (Gr. benthos – depth)- a system of organisms dwelling on the soil and in the soil of a waterbody bed.
Biocoenosis (Gr. bios – life + koinos – common) – a group of plants, animals and microorganisms, populating a given area of land or waterbody and specified by certain internal relations and adapted to the environment.
Biogenic elements – chemical elements of organisms that having certain biological functions.
Biomass – the accumulated mass of individuals of a specie, group of species or entire community (of plants, microorganisms and animals) per a unit of surface or volume of a habitat.
Biotope (Gr. bios – life + topos – place) - a part of land or waterbody surface with homogeneous conditions, occupied with a community of organisms (biocoenosis).
Clone (Gr. klon – offspring) – a number of subsequent generations of hereditarily homogeneous offsprings of an initital individual (plant, animal, microorganism) in the result of agamogenesis.
Cyclomorphosis – cyclic changes in the form, for instance, like the formation or disappearance of offshoots following the changes in water density in crustaceans..
Destruction (Lat. destruktio – disturbance) – destruction of a normal structure.
Ecosystem (Gr. oikos – habitation place) – an integral complex formed by living organisms and their environment (atmosphere, soil, water body, etc.), where organic and non-organic components are exchanging the matter, energy, and information.
Endemic forms (Gr. endemos – native) – species (genera, classes) of plants and animals typical for a relatively small area of land or water.
Eolion deposits – sand and clayey deposits formed by accumulation of wind-born particles.
Estuary (Lat. Aestuarium – low river outlet) – a funnel-shaped river outlet widening towards the sea.
Euryhalinious organism (Gr. eurys – wide + hals – salt) – an organism able to live in a wide range of salinity.
Eurythermobionts (Gr. eurys – wide + therm – temperature) – an organism able to live in a wide range of temperature.
Euthrophic water body (Gr. eu – good + trophe – food) – a water body abundant in biogenic elements.
Geological stage – a geological concept of subdividing the general stratigraphic scale, uniting the deposits formed during a geological age and corresponding to a definite geological development stage.
Halophiles (Gr. hals – salt + fileo – love) – salt-loving organisms.
Halophobes – (Gr. hals – salt + fobos – fear) – organisms avoiding high salt concentrations.
Holocene (Gr. holos – whole + kainos – common) – Postglacial Epoch or modern geological epoch, constituting the last, continuing anthropogenic (Quarternary) period of the geological history.
Hydrobiontes (hydor + bion – dweller) – a plant or animal, dwelling in water.
Hydrophytes (hydor + Gr. phyton – plant) – the plants, reproduction buds of which are located underwater; all the water plants.
Hyperhalinous (Gr. hyper – above + hals – salt) – of high salinity.
Malacofauna (Gr. malakion – mollusk) – the mollusk fauna
Meroplankton (Gr. meros – part + planktos – wandering) – a totality of organisms temporarily dwelling in the water column and unable to resist the transportation by water movement.
Mesotrophic – (Gr. mesos – middle and trophe – food) – a water body of medium trophicality.
Metamorphization – the process of significant change in the chemical composition of water.
Miocene (Gr. meion – less + kainos – common) – lower subdivision of Neogene.
Nectobenthos (Gr. nektos – floating + bentos – depth) - a totality of animals actively floating in the near-bottom waters.
Neolythic Age (Gr. neo – new + lithos – stone) – New Stone Age, the period from 8000-3000 BC till Common Era; transition from collecting and hunting to agriculture and cattle-breeding.
Oligohalinious (Gr. oligos – negligible + hals – salt) – of weak salinity.
Osmoregulation (Gr. osmos – push, pressure + Lat. regulo – directing). Physical, chemical and physiological processes that provide relatively stable osmotic pressure of internal environment (blood, lymph, hemolymph, intracellular fluid) of an organism. Osmoregulation assures the removal, sustaining and redistribution of water and salts (primarily, sodium chloride).
Paleohalinity (Gr. paleos – ancient + hals – salt) – the salinity of a water body in the course of past geological epochs.
Pelagic region (Gr. pelagos – sea) – the water column of oceans, seas and lakes as the biotope of pelagic organisms (plankton, nekton).
Phenotype (Gr. phaino – displaying) - a totality of all the features and characteristics of an organism, formed in the process of individual development.
Phytocoenosis – a plant community.
Plankton (Gr. planktos – wandering) – a group of organisms dwelling in the watercolumn and unable to resist transportation with water current.
Pliocene – the last epoch of Neogene period of the Earth geological history.
Population – a group of individuals belonging to the same specie, which occupies certain area for a long time and reproduces in many generations.
Primary production – assimilation of organic matter by autothrophs.
Quaternary (Period) – the third system of Cainozoic Era corresponding to the last period of geological history, subdivided into Pleistocene and Holocene.
Regression – in this case, slow recession of sea from shoreline.
Sedimentation (lat. sedimentum – sinking) - the sinking of small particles in a liquid or gas under the effect of gravitation or centrifugal forces.
Shelf – a flat part of underwater continental edge adjacent to the shore and having the same geological structure. Usually, the depth of the shelf edge is 100 to 200 m.
Solonchaks – soil type typical for steppe, semidesert or desert areas and containing water solved salts and 0,5-8% of humus.
Stratification – division into layers according to some property, such as salinity or temperature.
Stratigraphy – the section of historical geography, studying the succession of rock layers formation and their primary spatial relations..
Taxonomy – (Gr. taxis – arrangement, order, disposition + nomos – law) – a classification theory for animals and plants.
Transgression – in this case, slow rise of sea level and flooding of the shore..
Upwelling – elevation of the water from the bottom to the surface of a water body.
Uzboy – an ancient valley between Sarykamysh hollow and the Caspian sea.