
© 2000, Annual Reports of the Zoological Institute RAS.
Mikhail Yu. Punin, Tamara G. Markosova
Zoological Institute, Russian Academy of Sciences, Universitetskaya nab., 1, St. Petersburg, 199034, Russia
Histological, histochemical, electronmicroscopical and immunohistochemical investigations of last decades have demonstrated the presence of specialized cells in the digestive tract (its endodermal part) epithelium of many invertebrate species of different taxa (for references see reviews: Punin, 1986, 1993). While having some differences in morphology, these cells possess one common feature - they do not directly participate in digestive processes obviously playing a regulatory, integrating role. According to their gross and fine structure some of these "regulatory" cells can be regarded as endocrine while others closely correspond to neurons. At the same time many of them share structural features of both cell types. In better investigated invertebrate taxa (such as Insecta, Echinodermata, Mollusca, Annelida, Brachiopoda) one can conclude that these cells form their own complicated 'regulatory' system, a functional analog of well known gastro-intestinal diffuse endocrine system of vertebrates, first of all mammals (reviews: Ugolev, 1978; Solcia et al., 1981; Shahlamov & Makar', 1985; Pusyirev & Ivanova, 1992; and many others).
Evolution of Metazoan intestinal regulatory systems is of great interest for comparative and evolutionary histology, endocrinology and neurobiology. Essential information for better understanding of the origin and former evolutionary trends in the development of these systems can provide investigations of lower invertebrates. Being regarded as one of the most primitive bilaterally symmetric organisms closely related to ancestors of modern invertebrates (Ivanov & Mamkaev, 1973), turbellarian worms present in this aspect particular interest. It is also important to notice that in different groups of Turbellaria one can find significant varieties in digestive tract organization, starting from digestive parenchyma (Acoela) and finishing with well microanatomically and histologically differentiated intestine. That is why we can expect to reveal in intestinal regulatory system organization of the representatives of this Plathelminthes class some conditions most closely corresponding to initial one.
Unfortunately only scarce information on this topic is available from the literature. The presence of intraepithelial cells, reacting with the antiserum to FMRFamide, was demonstrated by immunohistochemical methods in the intestines of Gyratrix hermaphroditicus (Rhabdocoela) (Reuter et al., 1988) and Microstomum lineare (Macrostomidae) (Reuter et al., 1984). In the last species bovine pancreatic polypeptide (PP)- and vasotocin-immunoreactive cells were revealed too (Reuter & Palmberg, 1987). However, in M. lineare FMRFamide- and PP-like immunoreactivities were seemingly associated not with a separate cell type but with basal parts of gastrodermal glandular cells, and only antibodies to vasotocin reacted with different structures. At the same time intraepithelial neurons and nerve processes were revealed by means of electron microscopy (Reuter & Palmberg, 1987).
To update the present data on turbellarian intestinal regulatory cells we undertook comparative immunohistochemical and ultrastructural investigations of representatives of different (Tricladida, Polycladida, Macrostomida, Neorhabdocoela) orders of this class. Some preliminary data on polyclad and triclad flatworms were published (Punin & Markosova, 2000).
Practically in all investigated species of worms intestinal FMRFamide-immunoreactive elements were revealed. Nevertheless the observed patterns significantly differed from species to species (Fig. 1). In neorhabdocoelan
Astrothorynchus bifundus only rare intraepithelial processes running along the basal border of epithelium (gastrodermis) were detected, while in another worm species from this order -
Provortex karlingi - single small cells of closed type (situated in the basal part of gastrodermis and having no contact with intestinal lumen) were revealed too (Fig. 1, A). All these structures were observed in the beginning of intestine close to pharynx. Obviously they present the continuation of rich FMRFamide-like innervation of this organ.
In tricladid Procerodes littoralis only FMRFamide-immunoreactive processes are present (Fig. 1, B). Most of them have subgastrodermal localization and form a loose plexus. Single processes penetrate from this plexus into gastrodermis either ending between the basal parts of gastrodermal cells or running in the direction to intestinal lumen.
FMRFamide-immunoreactive cells and processes in another tricladid - Dendrocoelum lacteum - are localized mainly beneath the gastrodermis, where they form a very loose plexus partly enveloping branches of the intestine. Only single small cells of closed type are present in gastrodermis. Subgastrodermal plexus of Planaria torva intestine is developed much weaker while in gastrodermis besides closed type cells spindle-form cells of open type (with apical part reaching the gut lumen) were observed too (Fig. 1, C).
In contrast with previous species the intestinal FMRFamide-immunoreactive system of polycladid Notoplana atomata is well developed and formed by polymorphic cells with different localization relative to gastrodermis (Fig. 1, D). Both open type and closed type intraepithelial cells with basal processes are present. It is important to notice that intra- and subgastrodermal elements are obviously joined by processes in a common net. Some processes from parenchymal plexus not only penetrate gastrodermis but also reach the lumen of the gut.

Fig. 1. FMRFamide-immunoreactive elements in the intestine of (A) Provortex karlingi (Neorhabdocoela), (B) Procerodes littoralis, (C) Planaria torva (both - Tricladida), and (D) Notoplana atomata (Polycladida). gd - gastrodermis; par - parenchyma. This figure and following one demonstrate only the cell polymorphism and position of cells and processes relative to gastrodermis but not the frequency of their occurrence.
P. littoralis and both Notoplana species intestines also were investigated with antiserum to neurotensin (Fig. 2, A, B).
Intestinal neurotensin-immunoreactive elements revealed in P. littoralis are comparatively numerous and variable in their morphology (Fig. 2, A). Reacting with antiserum cell bodies were detected both in gastrodermis and beneath it. Subgastrodermal perikarya are small (less then 10 µm in length) and bipolar, possessing long varicose processes. These processes mainly form plexus enveloping the intestine while some of them penetrate gastrodermis and reach the gut lumen. Gastrodermal immunoreactive cells significantly differ in form and size. Elongated cells of open type usually posses one or two basal processes while cylindrical in form cells lack them. The cells of closed type are either small and stretch along the basal border of gastrodermis or (more rare) round in form and comparatively larger (about 15 µm in diameter). The cell processes partly form basal plexus, partly leave it and contact with enveloping the intestine subepithelial one. Perikarya of some cells are situated on the border of gastrodermis and parenchyma, a thin apical process runs in the direction to intestinal lumen, while another one leaves the gastrodermis.
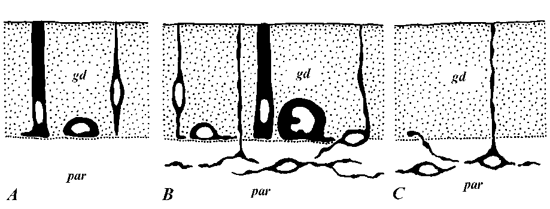
Fig. 2. Neurotensin- (A, B) and serotonin- (C) immunoreactive elements in the intestine of Procerodes littoralis (Triclada) (A, C) and Notoplana atomata (Polyclada) (B). For abbreviations see Fig. 1.
On the contrary, neurotensin-immunoreactive elements of N. Atomata have exclusively intraepithelial localization (Fig. 2, B). According to morphology they can be divided into several varieties. Two of them belong to open type cells either thin elongated with small nuclei or larger, cylindrical in form and often possessing two or three short basal outgrowths. Cells of the third variety are situated in the basal part of gastrodermis, lack cell processes and have no contact with intestinal lumen. While neurotensin-immunoreactive cells are rather numerous no basal gastrodermal plexus was revealed.
Comparatively similar pattern of neurotensin-immunoreactive structures was observed in N. humilis nevertheless no closed type cells were detected.
Finally immunostaining with antibodies to serotonin carried out on P. littoralis revealed only subgastrodermal plexus with rare processes penetrating between gastrodermal cells. Some of them run in the direction to intestinal lumen (Fig. 2, C).
Summarizing immunohistochemical data we can conclude that turbellarian worms possess a rather complicated intestinal regulatory system, containing different biologically active substances (at least demonstrating different immunoreactivities). Taking into consideration more or less developed intragastrodermal system of processes with typical varicosities and its contact with subgastrodermal (parenchymal) plexus we can regard it as a part of peripheral nervous system. Processes ending between gastrodermal cells seem to be effectory while others reaching the gut lumen may be receptory. Gastrodermal cells of open type are seemingly receptory-effectory in their nature.
At the same time intestinal neurotensin-immunoreactive system of N. atomata is exclusively intragastrodermal, most of cells lack processes and no plexus is observed. Obviously these features more correspond to elements of endocrine system. Probably some neurotensin-immunoreactive cells without processes detected in gastrodermis of P. littoralis also can be regarded as endocrine-like.
Electronmicroscopical investigations carried on Macrostomum sp. (Macrostomidae), P. torva and D. lacteum confirmed significantly complicated organization of turbellarian intestinal regulatory system.
Numerous single nerve processes and small nerve trunks were observed in muscle layer and parenchyma surrounding the intestine. Some of these processes with small electron dense granules about 80-120 nm in diameter and clear vesicles (40-60 nm) run near gastrodermis being separated from it only by very thin basal lamina. Intragastrodermal processes and single cell bodies sharing all features with nerve elements in parenchyma were also observed. Besides them processes and cell fragments with different from nerve as well as typical gastrodermal cells fine structure organization were revealed too.
In the gastrodermis of Macrostomum sp. these processes are very rare. They run near basal lamina and can be characterized by the presence of tightly packed small (about 60-90 nm in diameter) round granules with the content of moderate electron density.
In P. torva and D. lacteum intestine processes were observed more often. Also being situated in the basal part of gastrodermis they contain larger granules, round (with the diameter 200-230 nm) or elongated (165-200 x 400-450 nm). In the beginning of P. torva intestine close to the pharynx a gastrodermal cell partly submerged by its basal part into parenchyma was revealed. It contained numerous granules, similar in their size and form with the granules in described processes. These mentioned above structures seemingly represent endocrine-like elements.
Accepting conditions revealed in Turbellaria as closely related to initial ones we would like to suggest a following scheme of the origin and evolution of metazoan intestinal regulatory systems (Fig. 3).
Obviously the first elements of this system appeared practically simultaneously with the development of epithelialized intestine. These elements are represented mainly by neurons. Their variable position relative to the epithelium, the pattern we can observe in many of turbellarians, may indicate migration of nerve elements from epidermal layer to parenchyma (Ivanov & Mamkaev, 1973) continuing in the direction to intestinal wall and gastrodermis. Nevertheless even at this early stage in some turbellarian species the first rare intraepithelial endocrine-like cells may be already present.
In further intestinal regulatory systems become more complicated mainly due to appearance and development of morphologically different endocrine cells producing a wide spectrum of different regulatory substances.
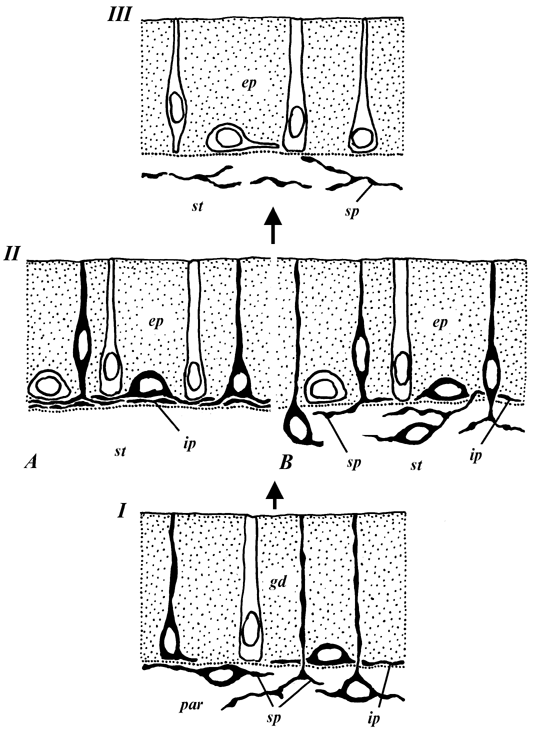
Fig. 3. Possible evolution of metazoan intestinal regulatory systems. I - initial stage (Turbellaria, Plathelminthes); II - regulatory systems in Asteroidea, Echinoidea (Echinodermata) and Brachiopoda (A), Gastropoda and Bivalvia (B), III - regulatory systems in Insecta (Protostomia) and Tunicata and Vertebrata (Deuterostomia). ep - epithelial layer; ip - intraepithelial plexus; sp - subepithelial (subgastrodermal in Turbellaria) plexus; st - subepithelial tissues. Black - neurons and nerve processes, white - endocrine (endocrine-like) cells. For other abbreviations see Fig. 1.
This condition can be observed in such invertebrate groups as polychaete and oligochaete worms (Annelida), brachiopods, gastropod and bivalve molluscs, sea stars and sea urchins (Echinodermata). Intraepithelial nerve elements remain and sometimes even get further development, forming vigorous basiepithelial layer. While in annelids and molluscs one can find intra- and subepithelial components joined by processes in a common system, in brachiopods and many echinoderms the intestinal nerve system in its localization is restricted by epithelium. Intraepithelial endocrine cells are often situated in direct contact with nerve processes.
Finally, in higher representatives of Protostomia - Insecta, and in Deuterostomia starting from Ascidiacea (Tunicata) the intraepithelial regulatory system consists exclusively of endocrine cells while nerve elements are localized beneath the epithelial layer. Even when nerve processes reach the bases of epithelial cells they do not have direct contact with them being separated by basal lamina.
The tissue source of intestinal endocrine cell development presents a question of important interest. Based on significant similarities in biochemistry of nerve and endocrine cells Pearse (1969) suggested that all endocrine elements derived from neuroectoderm (the concept of APUD-system). Nevertheless for mammals (Cheng & Leblond, 1974; and others) and insects (Endo et al., 1983) there is direct evidence for physiological regeneration of intestinal endocrine cells of some types from common epithelial source. These observations mean that at least partly intestinal endocrine cells have endodermal origin. Another confirmation for endodermal source for endocrine cell development is the presence of intestinal epithelial cell elements combining structural features of mucous and endocrine cells as well as endocrine and Paneth ones (Nebeyama, 1975; Puzyirev & Ivanova, 1986).
The excretory theory of secretion suggested by Ugolev (1985) also supposes plural sources for endocrine cell development. According to this concept both external and internal secretions are derived from unspecific excretion peculiar to all cells independently of their histogenetical origin. An important conclusion from this theory for us is the possibility of repeated appearance of internal secretion in different tissues.
In conclusion we would like to summarize the main positions of suggested intestinal regulatory systems evolution. Initially arising on the basis of nerve elements migrating to epithelial layer, these systems developed and complicated by repeated appearance in their composition of different endocrine cells having mainly endodermal origin. During the evolution segregation of endocrine and neuronal components obviously took place finally leading to a total disappearance of neurons and nerve processes from the epithelium. It is important to underline that the last process evidently went concurrently and independently in Protostomia and Deuterostomia.
Authors wish to thank Dr. S.A. Filimonova for technical assistance. The studies were carried out with financial support of the Russian Foundation for Basic Research (grant 99-04-49789).
Cheng, H. & C.P. Leblond. 1974. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. III: Enteroendocrine cells. Am. J. Anat. 141: 503-520.
Endo, Y., Sugihara, H., Fujita, T. & J. Nishiitsutsuji-Uwo. 1983. Kinetiks of columnar and endocrine cells in the cockroach midgut. Biomed. Res. 4: 51-60.
Ivanov, A.V. & Yu.V. Mamkaev. 1973. Resnichnye chervi: ikh proiskhozhdenie i evolyutsiya [Turbellarian worms: their origin and evolution]. Leningrad, Nauka. 221 pp. (In Russian).
Nebeyama, A. 1975. Presence of cell combining features of two different cell types in the colonic crypts and pyloric glands of the mouse. Am. J. Anat. 142: 471-483.
Pearse, A.G.E. 1969. The cytochemistry and ultrastructure of polypeptide hormone-producing cells of the APUD series and embryologic, physiologic and pathophysiologic implications of the concept. J. Histochem. Cytochem. 17: 303-313.
Punin, M.Yu. 1986. Endocrine-like cell elements in the intestinal epithelium of invertebrates. Tsitologiya 28 (10): 1035-1055. (In Russian).
Punin, M.Yu. 1993. The system of regulatory elements in the digestive tract epithelium of invertebrates. Tsitologiya 35 (2): 3-26. (In Russian).
Punin, M.Yu. & T.G. Markosova. 2000. FMRF-amide and neurotensin-immunoreactive elements in the intestine of some polyclad and triclad flatworms (Turbellaria). Tsitologiya 42 (5): 423-428.
Puzyirev, A.A. & V.F. Ivanova. 1986. "Mixed" glandulocytes in the epithelium of duodenum of some vertebrates and man. Arhiv Anat. Gistol. Embriol. 90 (4): 48-54. (In Russian).
Puzyirev, A.A. & V.F. Ivanova. 1992. Gastroenteropancreatic system (development, structure, regeneration). Morphologiya 102 (1): 5-28. (In Russian).
Reuter, M. & I. Palmberg. 1987. An ultrastructural and immunocytochemical study of gastrodermal cell types in Microstomum lineare (Turbellaria, Macrostomidae). Acta zool., Stockh. 68: 153-163.
Reuter, M., Karhi, T. & L.P.C. Schot. 1984. Immunocytochemical demonstration of peptidergic neurons in the central and peripheral nervous system of the flatworm Microstomum lineare with antiserum to FMRF-amide. Cell Tissue Res. 238: 431-436.
Reuter, M., Lehtonen, M. & M. Wikgren. 1988. Immunocytochemical evidence of neuroactive substances in flatworms of different taxa - a comparison. Acta zool., Stockh. 69: 29-37.
Shahlamov, V.A. & V.I. Makar'. 1985. Enteroendocrine cells, their structure and function. Arhiv Anat. Gistol. Embriol. 89 (9): 7-17. (In Russian).
Solcia, T., Capella, C., Buffa, R., Usellini, L., Fiocca, R. & F. Sessa. 1981. Endocrine cells of the digestive system. In: Physiology of gastrointestinal tract. Vol. 1. pp. 39-58. New York, Raven Press.
Ugolev, A.M. 1978. Enterinovaya (kishechnaya gormonal'naya) sistema [Enteric (intestinal hormonal) system]. Leningrad, Nauka. 314 pp. (In Russian.)
Ugolev, A.M. 1985. Evolyutsiya pishchevareniya i printsypy evolyutsii funktsiy: elementy sovremennogo funktsionalizma [Evolution of digestion and principals of evolution of functions: elements of modern functionalism]. Leningrad, Nauka. 544 pp. (In Russian).