© 2000, Annual Reports of the Zoological Institute RAS.
Andrey A. Przhiboro
Zoological Institute, Russian Academy of Sciences, Universitetskaya nab., 1, St. Petersburg, 199034, Russia
Macrophyte stands of the shallow littoral zone of lakes are characterized by a very high primary and secondary productivity among the stagnant water habitats (Brinkhurst, 1974; Wetzel, 1975; Brinson et al., 1981). However, this zone is ignored very often in both hydrobiological and entomological investigations in particular for the methodical difficulties of sampling. The faunal composition of some groups inhabiting lake littoral such as non-chironomid Diptera (Insecta) and their role in benthic communities are not investigated either.
For the present study the materials collected by the author on 10 small lakes in the North-West of Russia were used. Six lakes were investigated in detail: lakes Krivoe and Krugloe (66°21'N 33°35'E; Loukhi Distr. of Karelia), Pionerskoe and Pridorozhnoe (60°18'N 29°17'E; Vyborg Distr. of Leningrad Prov.), Anninskoe and Anisimovo (56°12'N 28°40'E; Sebezh Distr. of Pskov Prov.). On every lake, the benthic quantitative samples (S = 0.033-0.06 m2) were collected in the littoral zone to the water depth of 1-1.5 m and in the zone of water line. Series of samples were taken 3-5 times during the season on the model sites at each of 6 lakes. In littoral, the folding circle sampler with attached net bag was mainly used (all samples were taken to a depth of 7 cm and more from the bottom surface). The vertical distribution of macrobenthos in bottom substrata was studied with Mordukhai-Boltovskoj core sampler. All the samples were washed on sieves (the least 0.25 mm mesh), then the benthos was extracted by the flotation in strong solution of NaCl, combined with the hand-sorting of coarse fraction. A total of 331 quantitative and nearly 200 qualitative samples have been processed. The identifications of Diptera were made using the imagines reared from the separate larvae and pupae.
The dense vegetation with predominating emergent macrophytes is located in littoral only in the eutrophic lakes of Pskov Prov. (Anninskoe and Anisimovo), at a depth of 0.1 to 0.7 m, in places - to 1 m. In this zone on many sites protected from the wave action the roots of emergent monocotyledones (Carex rostrata, Scirpus lacustris, Acorus calamus, Phragmites australis, Glyceria maxima, Typha spp., etc.) constitute dense silted turf up to 10 cm thick accumulating high quantity of plant remains. Organic components comprise most of sediments in this layer, aside from the interlacement of roots. Such sites are 5 to 20 m in width to the lower edge of turf and correspond to 25-100% of the shore line length of a lake.
Benthic larvae of several species of Diptera live only in this habitat of the littoral zone and occur exclusively in the layer of turf. Specifically, they are completely absent in qualitative samples taken with net and abundant in quantitative samples. They are: Erioptera squalida (and possibly, E. flavata; Limoniidae), Chrysops rufipes (Tabanidae), Melanogaster aerosa (Syrphidae), and Notiphila spp. (Ephydridae; reared imagines belong to N. aquatica and N. nigricornis). Besides, the larvae of Palpomyia tibialis (Ceratopogonidae) are restricted to the layer of turf in this habitat, they are not found on the surface of the turf and above, as distinguished from all the other Ceratopogonidae. However, single larvae of P. tibialis occur on the sites without turf.
Larvae of E. squalida, Ch. rufipes, P. tibialis, and usually those of Notiphila are numerous on the sites with predominant Carex rostrata, Scirpus lacustris, Glyceria maxima, Typha spp., while M. aerosa are small in number. Larvae of Ch. rufipes, P. tibialis and Notiphila are numerous also in the association of Sagittaria sagittifolia and Potamogeton natans, whereas larvae of Erioptera and Melanogaster are not found in these stands. Thus, Erioptera gives a unique example of metapneustic limoniid larvae inhabiting the bottom up to 1 m beneath the water surface, that was not recorded earlier. All the species except P. tibialis were not found in the pure stands of Numphar luteum, Sparganium spp., Phragmites australis, Scolochloa festucacea, Acorus calamus.
The respiration, feeding and pupation were studied for these species or ecologically similar forms of the same genera (Dette, 1916; Varley, 1937; Hinton, 1953; Hartley, 1961; Hoolihan, 1969; Busacca & Foote, 1978; Deonier et al., 1978; Lutta & Bykova, 1982; Andreeva, 1990). According to these papers and to the author's data, the turf-inhabiting larvae except P. tibialis share 3 common traits:
The complex of root-piercing larvae developing in common microhabitat was first mentioned by Edwards (1919) for the shore zone of a pool and was not investigated later. Until the present time it was not recorded for the littoral of lakes.
In addition to the above-mentioned genera, a rather restricted number of insects have aquatic immatures, which obtain oxygen from vessels of plants. Among them are:
All these insects except the subtropical Lissorhoptrus and mentioned Ceratopogonidae are usual in the littoral zone of lakes Anninskoe and Anisimovo, and yet only Donacia inhabit the layer of turf (see below), others occupy other microhabitats, being different.
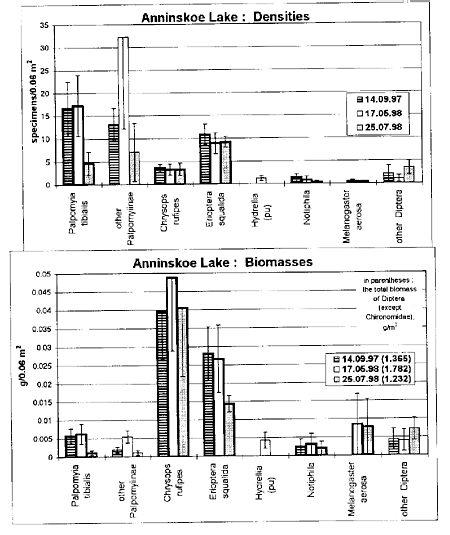
The total biomass of the turf-inhabiting Diptera (including P. tibialis) was in the range from 0.3 to 3.7 g/m2 in the littoral habitat described above. Typically, Erioptera and Chrysops account for more than 70% of the biomass, whereas P. tibialis and Erioptera predominate in numbers. The seasonal changes of densities and biomasses were studied on two model sites (Figs 1, 2). The biomasses of all the taxa decreased by July as compared to May in connection with the completion of development. The highest densities and biomasses were observed mainly in May (Figs 1, 2). The values of occurrence in samples were very high (more than 90%; n=15) for P. tibialis, E. squalida and Ch. rufipes on both of sites, and for Notiphila - only at Anisimovo Lake. E. squalida shows the least values of standard error of density (in % of mean) among all the taxa of non-chironomid Diptera (Figs 1, 2).

Fig. 1. Seasonal changes of mean densities and biomasses (±1 standard error, n=5) of non-chironomid Diptera on the model site in the littoral zone of Anninskoe Lake at depths 0.2-0.7 m (plant associations: Carex rostrata / Acorus calamus, Scirpus lacustris). The columns representing abundance values of turf inhabitants are with thick outlines. All the values of biomass represent wet weight.
On the model site in Anisimovo Lake, the total biomass during the season was 1.2-1.9 times higher than on the model site in Anninskoe Lake (Table 1). In Anisimovo, the biomasses of all turf inhabitants except M. aerosa were generally 1.3-6 times as high as in Anninskoe (Figs 1, 2). On both model sites the numbers and biomasses of all the taxa of Diptera are distributed non-uniformly among the main plant associations.
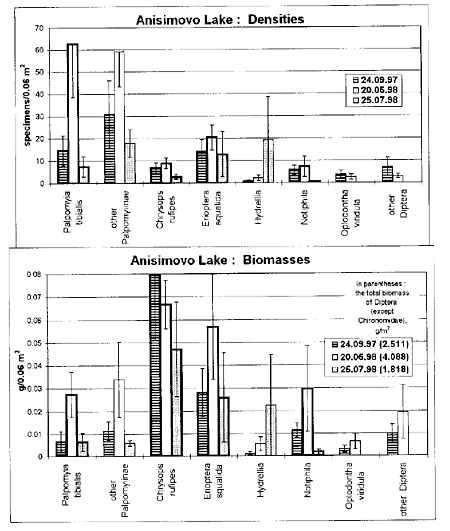
Fig. 2. Seasonal changes of mean densities and biomasses (±1 standard error, n=5) of non-chironomid Diptera on the model site in the littoral zone of Anisimovo Lake at depths 0.2-1.0 m (plant associations: Potamogeton natans / Sagittaria sagittifolia, Glyceria maxima, Carex rostrata, Typha latifolia / Stratiotes aloides). The designations as in Fig. 1.
On the most of the sites investigated the biomass of turf inhabitants comprises 70-95% of the biomass of non-chironomid Diptera, and 0.5-14% of the total biomass of macrobenthos (e.g., Table 1). The last value was from 5 to 20 g/m2 for these sites of the low-eutrophic lake Anninskoe and usually 150-350 g/m2 for high-eutrophic lake Anisimovo.
The vertical distribution of larvae in bottom substrata was studied in littoral on the model site in Anninskoe Lake:
Table 1. The biomass of the turf inhabitants (TI) and its proportion in the total biomass of macrobenthos in the littoral on the model sites
|
Lake, |
Anninskoe, 20-70 cm |
Anisimovo, 20-100 cm |
||||
|
Date |
14.09.97 |
17.05.98 |
25.07.98 |
24.09.97 |
20.05.98 |
25.07.98 |
|
Biomass of Diptera-TI, |
1.261 |
1.550 |
1.091 |
2.090 |
3.007 |
1.350 |
|
% of Diptera-TI in biomass of |
92.4 |
87.0 |
88.5 |
83.2 |
73.5 |
74.2 |
|
% of Diptera-TI in total |
6.8 |
- |
6.9 |
0.6 |
- |
0.7 |
|
The same, taking no account |
6.8 |
- |
6.9 |
3.0 |
- |
2.1 |
|
Total proportion of TI |
6.8 |
- |
6.9 |
1.2 |
- |
4.1 |
|
The same, taking no account |
6.8 |
- |
6.9 |
5.6 |
- |
10.8 |
|
Biomass of macrobenthos, |
18.60 |
- |
15.90 |
337.42 |
- |
183.68 |
Note. All the values of biomass represent wet weight; for each date n=5.
All abundant taxa of turf inhabitants except for Erioptera demonstrated the highest values of density and biomass at the least depths of the littoral zone (20-30 cm), i.e. close by the zone of water line. The least values were observed near the lower edge of turf (70-100 cm). Besides, all turf-inhabiting Diptera occur in the zone of water line on the sites with well-developed cover of monocotyledones, but their mean biomass was in the range from 0.1 to 0.9 g/m2, that was usually 3-12 times as low as in littoral on the same site, and always constituted not more than 1/3 of the total biomass of non-chironomid Diptera.
The assemblage of turf inhabitants divides sharply on the place of pupation. The larvae of P. tibialis, Ch. rufipes and M. aerosa migrate to the zone of water line for pupation, pupa respires with oxygen of the air. All the pupae of these species were collected only in the zone of water line. In contrast, Notiphila and Erioptera pupate in the same habitat, where they develop, pupa respires through the aerenchyma like larvae (Hoolihan, 1969; Busacca & Foote, 1978; original data). Thus, Notiphila and E. squalida are among a few of benthic Diptera to pupate at a depth of 0.5-0.8 m under the water surface, and the only ones not to be apneustic. In laboratory, the larvae of Erioptera were not capable to pupate without roots of live plants. Imagines of P. tibialis emerged from middle June to middle August, E. squalida - during July, Ch. rufipes - from early July to early August, Notiphila spp. - from late May up to the end of July. According to our data, P. tibialis is univoltine, the larvae require thermoreactivation to pupate. The other turf inhabitants are univoltine, a part of the larvae of E. squalida possibly develops during 2 years. Overwintering of all the species takes place as larvae.
The turf-inhabiting Diptera constitute the synusia, isolated clearly from the other littoral macrobenthos, i.e. the detached part of community, both spatially and ecologically (following the definition of synusia given in: Mirkin et al., 1989). A high degree of isolation is due to the probable very low accessibility of the turf inhabitants to the potential predators and parasitoids. No consumers of turf-inhabiting larvae were registered. By this means this synusia appears to be more isolated from the potential consumers than the phytophages mining the above-ground parts of plants on the same sites: the latter are cryptobiontic as well, and many of them pupate inside the host plant (e.g., Hydrellia, Cordylura), but they frequently become infected by parasitoids.
Apart from Diptera, Donacia (Chrysomelidae) belong to the subcommunity of the turf inhabitants. On some sites, Donacia constituted more than a half of the total biomass of the turf-inhabiting macrobenthos (e.g., Table 1). The larvae and pupae of Donacia spp. develop in the same layer of bottom as Diptera, but differ in trophic relations as they feed on the roots of plants being used for the respiration (Ogloblin & Medvedev, 1971). Moreover, Donacia spp. use the more wide spectrum of host plants, and the genus is not confined to the habitat of turf at the lakes being studied.
The synusia being considered is poorly developed or absent in the oligo- and mesotrophic lakes of Karelia and Leningrad province (see above). Only the larvae of Erioptera aff.flavata are common, they inhabit the sites with sparse stands of Carex spp. at 5-30 cm depth (the biomass was less than 0.2 g/m2). Notiphila and Donacia were solitary.
The synusia of turf inhabitants bears taxonomical similarities to the population of semiaquatic habitats (e.g., moist grasslands, upper intertidal zone of seas) and has no analogues among the benthic communities of standing waters.
The studies were carried out with financial support of the Russian Foundation for Basic Research (grant 96-15-97875).
Andreeva, R.V. 1990. Opredelitel' lichinok slepnei: evropeiskaya chast' SSSR, Kavkaz, Srednyaya Aziya. [Keys to the larvae of horse-flies: European part of the USSR, Caucasus, Middle Asia]. Kiev, Naukova Dumka. 172 pp. (In Russian).
Brinkhurst, R.O. 1974. The benthos of lakes. London/Colchester/Beccles, W. Clowes and Sons. 190 pp.
Brinson, M.M., Lugo, A.E. & S. Brown. 1981. Primary productivity, decomposition and consumer activity in freshwater wetlands. Ann. Rev. Ecol. Syst. 12: 123-161.
Busacca, J.D. & B.A. Foote. 1978. Biology and immature stages of two species of Notiphila, with notes on other shore flies occuring in cattail marshes (Diptera: Ephydridae). Ann. ent. Soc. Am. 71 (3): 457-466.
Deonier, D.L., Mathis, W.N. & J.T. Regensburg. 1978. Natural history and life-cycle stages of Notiphila carinata (Diptera: Ephydridae). Proc. biol. Soc. Wash. 91 (4): 798-814.
Dette, E. 1916. Über die Metamorphose von Trichosticha flavescens. Zool. Jb. (Abt. Syst.) 39: 417-443.
Edwards, F.W. 1919. The larva and pupa of Taeniorhynchus richardii Fic. Entomologist's mon. Mag. 5: 83-88.
Hartley, J.C. 1961. A taxonomic account of the larvae of some British Syrphidae. Proc. zool. Soc. Lond. 136 (4): 505-573.
Hinton, H.E. 1953. Some adaptations of insects to environments that are alternately dry and flooded, with seem notes on the habits of the Stratiomyidae. Trans. Soc. Br. Ent. 11: 209-227.
Hoolihan, D.F. 1969. The structure and behaviour of Notiphila riparia and Erioptera squalida, two root-piercing insects. Proc. zool. Soc. Lond. 159 (2): 249-267.
Isely, D. & H.H. Schwardt. 1930. The tracheal system of the larva of Lissorhoptrus simplex. Ann. ent. Soc. Am. 23: 149-152.
Lutta A.S. & H.I. Bykova. 1982. Slepni (sem. Tabanidae) Evropeiskogo Severa SSSR [The horse-flies (Tabanidae) of the European North of the USSR]. Leningrad, Nauka. 184 pp. (In Russian).
Mirkin, B.M., Rosenberg, G.S. & L.G. Naumova. 1989. Slovar' ponyatij i terminov sovremennoi fitotsenologii [The dictionary of concepts and terms of modern phytocoenology]. Moskva, Nauka. 223 pp. (In Russian).
Nilsson, A.N. 1996. Coleoptera Noteridae, burrowing water beetles. In: Aquatic insects of North Europe - a taxonomic handbook. Vol. 1. pp. 139-143. Stenstrup, Apollo Books.
Ogloblin, L.A. & L.N. Medvedev. 1971. Lichinki zhukov-listoedov (Coleoptera, Chrysomelidae) evropeiskoi chasti SSSR. [The larvae of Chrysomelidae (Coleoptera) of the European part of the USSR]. Leningrad, Nauka. 123 pp. (In Russian.)
Varley, G.C. 1937. Aquatic insect larvae, which obtain oxygen from the roots of plants. Proc. R. ent. Soc. Lond. (A) 12: 55-60.
Wetzel, R.G. 1975. Edgardo Baldi memorial lecture. Land-water interfaces: metabolic and limnological regulators. Verh. int. Verein. theor. angew. Limnol. 24: 6-24.