© 2000, Annual Reports of the Zoological Institute RAS.
Anatoly A. Petrov
Zoological Institute, Russian Academy of Sciences, Universitetskaya nab., 1, St. Petersburg, 199034, Russia
The cyclical changes in the structure of the reproductive system seem to be fairly common in the monocelidid turbellarians. Evidently, it is the high variability of the features of genital organs, which is the primary cause of the numerous errors and confusion, frequently arising during the species diagnostics. However, despite the great significance of the reproductive transformations for the taxonomy of the family, only a few comprehensive works (Eropkina, 1977, Fleming & Burt, 1978a, 1978b) and several sporadic considerations of the problem have been published. Apparently, thorough investigation of the monocelidid life cycles is neccessary for the further elucidation of the problem.
In this account, I offer a preliminary analysis of the reproductive cycles of six common monocelidid species, based upon my own observations as well as the data, obtained from the literature.
The material for the study was collected in Kandalaksha Gulf of the White Sea at different times of the year (species: Archilopsis spinosa, A. unipunctata, Monocelis fusca, M. lineata, and Promonotus schultzei). Living flatworms have been studied both in natural and laboratory conditions. Specimens were maintained in glass dishes at a temperature of 10 °C from May to August and from October to February. Supplementary information has been received from the material, retained in the collection of the Zoological Institute, RAS. Specimens of Ectocotyla hirudo were obtained from the external surfaces of the crustacean Parapagurus pilosimanus in the Indian Ocean (33° 28' S, 44° 22' E, depth - 850-870 m) during the seventeenth voyage of the 'Vityaz-II'.
The investigation of E. hirudo provides the most remarkable example of the reproductive transformations, for in this flatworm the accumulation of the complicate egg and early stages of embryonic development occur directly within the gastric cavity. Fleming & Burt (1978b) in their comprehensive work, although explicitly studied the process of egg formation, paid no attention to the morphological transformations that accompany the reproductive cycle. However, even the simple comparison of the species descriptions, found in the literature (Hyman, 1944; Westblad, 1952; Karling, 1966), shows that certain radical changes in the morphology of the animal must take place during the spawning season.
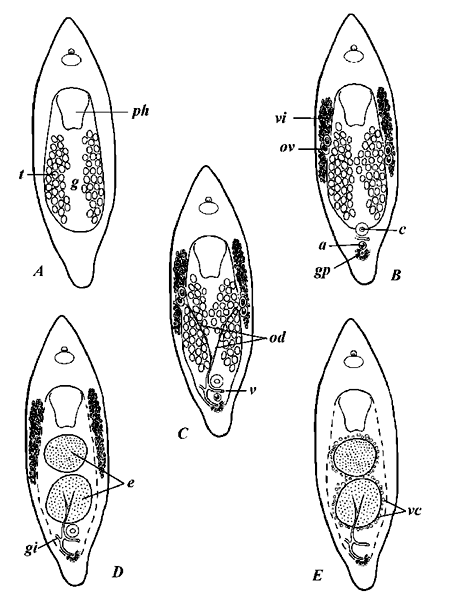
Five stages are discerned within the reproductive cycle (Fig. 1). The first is marked by the presence of the testicular follicles, with other reproductive organs either completely missing or extremely arrested in development (Fig. 1A). During the next period, female gonads (germaria and vitellaria) become distinguishable. The differentiation of the female gonads occurs with the appearance of the male copulatory organ and accessory "prostatoid" vesicle (Fig. 1B); the latter consists of a muscular sac, provided with hollow spicule, and represents a specific trait of the subfamily Minoninae. At that stage, gonoducts are entirely absent. The principal characteristic of the next period is development of the oviducts and genito-intestinal canal; instead of removing the excess sperm, the latter fulfills a function of transportation of the fertilized eggs to the gastric cavity (Fig. 1C). The bursa is lacking and the sperm is stored directly in the oviducts, where fertilization takes place; shortly afterward the eggs through the genito-intestinal duct fall into the gut lumen (Fleming & Burt, 1978b). At the beginning of the next period, the autolytic processes in gastrodermis are initiated (Fig. 1D). Gastrodermis breaks down and the gut lumen as a whole achieves an appearance, similar to the digestive parenchyma of the Acoela. As a consequence of autolysis, a connection between vitellaria and digestive cavity appears and the vitellaria pass contents into gut. Accordingly, the accumulation of yolk and the formation of ectolecithal egg take place within the gastric cavity. At this point, most part of the gonoducts becomes obliterated, excluding their distal fraction with a gonopore and the genito-intestinal canal (Fig. 1E). Those remaining possibly participate in the final stages of egg production, being provided with shell glands. Eventually, after losing all traces of the gonoducts, the reproductive cycle returns to its starting point (Fig. 1A).
It would be reasonable to suppose that the use of the gut as a brooding chamber is related to the ectocommensal lifestyle. The representatives of Ectocotyla dwell in the branchial cavities and around the mouthparts of the decapods. It is obvious, that the young must be able to attach themselves to the host's carapace just after emerging from the egg. For this reason, the hatchlings are produced active and capable of seeking an appropriate habitat. This could account for prolonged period of embryonic development and the large relative size of the egg capsule (mature specimens of E. hirudo vary from 0.9 to 1.4 mm in body length, while their eggs measure up to 0.5 mm in diameter). Apparently, the only body cavity of appropriate space to retain fully-grown eggs is the gut lumen.

Fig. 1. Diagram showing reproductive stages of Ectocotyla hirudo. A - E - stages of the reproductive cycle; a - accessory vesicle; c - copulatory organ; e - fertilized eggs; g - gut lumen; gi - genito-intestinal canal; gp - gonopore; od - oviducts; ov - ovary; t - testes; v - vagina; vi - vitellaries.
Similar function of the gut and supposedly similar reproductive cycles were also described for Peraclistus (Fleming & Burt, 1978a) and Duploperaclistus (Martens, 1983). Undoubtedly, these genera are closely related to Ectocotyla and all three should be united in a monophylum.
Ectocotyla and related genera are the most striking but by no means the only examples amongst the turbellarians of such uncommon role of the gut. In the Neoophora outside the Proseriata, we also know several species in which the eggs or embryos are transported secondarily through the intestine (for example, Bresslauilla relicta - Reisinger, 1929; Baicalellia evelinae - Marcus, 1946; and Ethmorhynchus anophthalmus - Meixner, 1938). Moreover, Bresslauilla relicta shows unequivocally that the female gonoducts are reduced in connection with the secondary development of eggs in the intestine (Reisinger, 1929).
The reproductive changes of the examined monocelidids from the White Sea have a seasonal character. The following consecutive periods can be recognized in the reproductive cycles of all investigated nothern species: the spawning (May-July), the reduction of the genital organs (August-October), the formation of gonads and the maturation of the male germ cells (November-February), the ripening of the ovo- and vitellocytes (March-April). All organs of the female reproductive system become undistingishable during the autumn; the most variable are the gonoducts, which could be of different arrangement and shape during the different seasons of the year. The most spectacular is the transformation of the female ducts and the bursal organs in Monocelis. The representatives of this genus possess bursae of resorbent function (i.e. the function of the eradication of excess sperm). As the study of the serial sections indicates, the bursae originate by cell dissociation in parenchyma independently of gonoducts. In the early stages, bursae exist as schizocoele lacunae, later they get their own epithelial lining. Generally, there are one main bursal cavity and 2-5 accessory diminutive bursae. After copulation the cells of the bursa (in the vicinity of the female duct) elongate and extend to the duct epithelium, thus creating transient passage, through which the sperm is transferred. The occurence of the sperm in bursal lumen probably stimulates a resorption of this provisional canal; subsequently, the elongated cells of the bursal epithelium, adjacent to the gut, give rise to the genito-intestinal duct, through which the sperm is discharged into the instestine.
There is a definite correlation between the changes in the reproductive apparatus and those in the digestive system. Highest activity and the largest sizes of the gut cells were observed from August to February. In the spring, considerable transformation in the structure of the digestive system occurs: the cells of the gut epithelium diminish in volume and the gastrodermis attains a parenchymatous constitution. The reduction in size of the digestive cells depends on decreasing, and then full disappearance of the inclusions of nutritious substances (probably, fats). Apparently, the digestive cells serve as energetic resources for vitello- and ovogenesis, and later, possibly, for reorganisation of the reproductive system after the spawning.
In M. fusca the dissolution of the vitellaria and gonoducts is accompanied by an autotomy of the hindmost part of the body (from mid-October to December). The autotomy begins by circular furrow at the base of the pharynx, which has resulted from the constriction of the pharyngeal sphincter muscle. After 3-4 days an immobilized last part of the body is cast off, while the anterior fragment (which is about 1/3 of the body length) remains active over a prolonged period of time. Therefore, the most components of the reproductive and digestive systems (excluding several testicular follicles) become lost. Whether the flatworm can replace missing parts of the body by growing them anew or these changes have a degenerative character is not clear.
The observations, presented in this account, lead to the following preliminary conclusions:
The studies were carried out with financial support of the Russian Foundation for Basic Research (grants 99-04-49783 and 99-04-49807).
Eropkina, E.M. 1977. Seasonal differencies in the morphology or reproductive and digestive systems of turbellarian Monocelis lineata. In: Biologiya severnykh morei evropeiskoi chasti SSSR [Biology of the northern seas in European part of USSR]. pp. 70-76. Apatity. (In Russian).
Fleming, L.C. & M.D.B. Burt. 1978a. On the genus Peraclistus (Turbellaria, Proseriata), with redescription of P.oofagus (Friedman). Zool. Scr. 7: 81-84.
Fleming, L.C. & M.D.B. Burt. 1978b. Revision of the Turbellarian genus Ectocotyla (Seriata, Monocelididae) associated with the crabs Chionoecetes opilio and Hyas araneus. J. Fish. Res. Bd Can. 35: 1223-1233.
Hyman, L.H. 1944. Marine Turbellaria from the Atlantic coast of North America. Am. Mus. Novit. 1266: 1-15.
Karling, T.G. 1966. Marine Turbellaria from the Pacific coast of North America. IV: Coelogynoporidae and Monocelididae. Ark. Zool. 18: 493-528.
Marcus, E. 1946. Sôbre Turbellaria brasileiros. Boln Fac. Filos. Ciênc. Univ. S. Paulo (Zool.) 11: 5-254.
Martens, P.M. 1983. Three new species of Minoninae (Turbellaria, Proseriata, Monocelididae) from the North Sea, with remarks on the taxonomy of the subfamily. Zool. Scr. 12: 153-160.
Meixner, J. 1938. Turbellaria (Strudelwürmer). 1: Allgemeiner Teil. In: Die Tierwelt der Nord und Ostsee. Bd. 4b. Leipzig, Akad. Verl. 146 S.
Reisinger, E. 1929. Zum Ductus-Genito-Intestinalis Problem. I: Über primäre Geschlechtstrakt-Darmverbindungen bei rhabdocoelen Turbellarien. Z. Morph. Ökol. Tiere, 16 (1-2): 49-73.
Westblad, E. 1952. Turbellaria (excl. Kalyptorhynchia). Further zool. Results swed. Antarct. Exped. 1901-1903 4 (8): 1-55.