© 2000, Annual Reports of the Zoological Institute RAS.
Sergey D. Grebelnyi
Zoological Institute, Russian Academy of Sciences, Universitetskaya nab., 1, St. Petersburg, 199034, Russia
The unisexual reproduction occurs in quite different animal groups, though the all-female species are not numerous in nearly all the taxa. The majority of unisexual species which have been discovered by now are supposed to be parthenogenetic, and this seems to be true. However, in a few species some other kinds of unisexual reproduction and inheritance have been recorded.
Avoidance of the normal sexual reproduction can produce the genetic effect of different sorts. Being dependent on particular mechanisms of preservation of the somatic parental chromosomal number in offspring, it can vary from inheritance of only maternal features to complete substitution of paternal for maternal ones. Anyway, it seems to be obvious, that any unusual rearrangement of the genetic material, while reproducing, abruptly changes the characteristic features of the 'mendelian population', which are considered to be the base of the widely accepted concept of speciation.
Parthenogenesis. During parthenogenesis the juvenile originates from an egg without any participation of spermatozoa. Fertilization does not occur, that is why in such a case the complete (non reductional) diploid or polyploid chromosome set of somatic cells is to be preserved in the egg. In natural populations the parthenogenesis is always connected with deep disintegration of meiosis that leads to a complete stoppage of genetic recombination, so that the maternal characters are inherited by the offspring without any c the population an opportunity not to spend 50% of environmental resources hanges.
When discussing the origination of parthenogenetic forms and inevitable competitive interactions of them with their closest bisexual relatives, one quite often pays attention to the 'double advantage' of parthenogenesis (Maynard Smith, 1971, 1978) that gives on males. According to another, may be more reasoned point of view, the advantages of parthenogenetic populations could be caused by their higher heterozygosity and uniformity much more than by higher rate of breeding without males (Suomalainen, 1969; Suomalainen et al., 1973, 1976; Grant, 1977; and many others).
The parthenogenetic-like effect is also produced by some other secondary aberrations of sexual reproduction, they are: gynogenesis, hybridogenesis and androgenesis. For reproduction of gynogenetic and hybridogenetic unisexual all-female forms the males of closely related bisexual species are needed. Androgenesis as any kind of multiplication by means of egg produced by a feminine gonad cannot proceed without females. Therefore the only one undoubtedly androgenetic clonal organism known in nature is a hermaphroditic clam (to be discussed below).
Gynogenesis. Gynogenesis is a development of unfertilized eggs that proceeds only after stimulation of them by sperm. Spermatozoid penetrates egg, but soon, the male pronucleus is to be eliminated. Further development of the embryo is controlled only by maternal genome and leads to production of all-female offspring.
The widely known example of gynogenesis is Carassius auratus gibelio, in addition to that the phenomenon was also discovered in diploid and triploid races of small viviparous tropical fishes of the genera Poecilia and Poeciliopsis (Hubbs & Hubbs, 1932; Berg, 1947; Golovinskaja & Romashov, 1947; Cherfas, in Kirpichnikov, 1987), in some atherin fishes Menidia and Cobitis (Vasil'ev & Vasil'eva, 1982; Vasil'ev, 1985; Echelle & Mosier, 1981, 1982; Echelle et al., 1983), in triploid races of the amphibian genus Ambystoma (MacGregor & Uzzell, 1964; Cuellar, 1974), and in insects (triploid race of beetle Ptinus mobilis, the reproduction of which depends on the presence of males of the diploid conspecific race: Sanderson, 1960).
Hybridogenesis. During hybridogenesis, the offspring originates from fertilized eggs, that is the principal difference between hybridogenesis and gynogenesis. The genes of a father being brought by spermatozoid reveal themselves in phenotype. As well as in the cases mentioned above, the offspring is also all-female, but of a hybrid constitution, because it inherited chromosomes from both parents, that was proved by crosses of individuals with marker alleles. However, during the initial stage of oogenesis the father's chromosomes are eliminated and only the mother's ones are to be retained in a mature egg. Therefore each subsequent generation can be produced only after one haploid set of chromosomes is again borrowed from a male of the related species. This kind of reproduction was suggested to be called the 'creditogenesis' (Borkin & Darevsky, 1980).
The phenomenon of hybridogenesis was most completely investigated in small fishes Poeciliopsis (Schultz, 1967, 1969, 1977; Vrijenhoek, 1972). These are diploid all-female hybrid populations that originated from different species of the genus. For reproduction they need males of related bisexual species. Strong evidence of hybridogenesis was discovered in the frog Rana esculenta. In reality, this well known European species is a permanent hybrid of R. lessonae and R. ridibunda (Berger, 1967; Borkin et al., 1987; Vinogradov et al., 1990; Lada et al., 1995).
Androgenesis. As well as gynogenesis, the androgenesis in nature can serve as one of the ways of cloning or multiplication of identical genotypes. It may be called mirroring of parthenogenesis. The offspring originates from the eggs stimulated by sperm. In the course of meiosis nucleus of the egg produces two polar bodies, then both of them are moved out from the cell. Only chromosomes derived from male pronucleus constitute the metaphase of the first cleavage of zygote, whereas the female pronucleus which during the normal sexual reproduction is to fuse with the male one, is absent at all. The development of embryo is controlled only by the genes introduced by spermatozoid. In the case of natural androgenesis spermatozoid involves non-reductional set of chromosomes, either diploid or triploid (in triploid species).
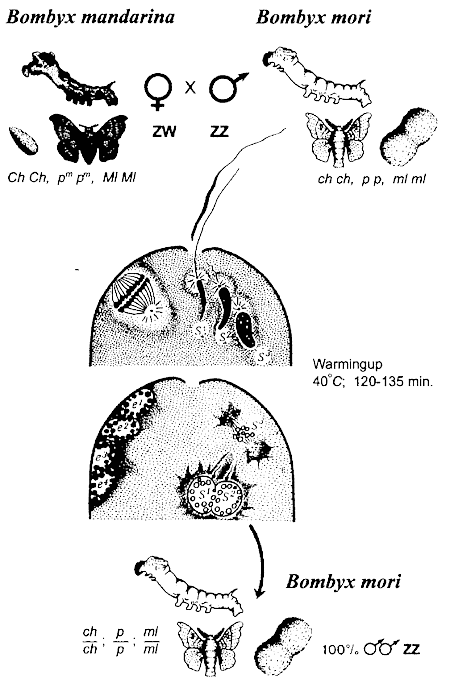
The possibility of androgenetic development was so obvious to some experienced geneticists that they firstly managed to demonstrate this wonderful phenomenon in experiments on the silkworm (Hasimoto, 1934; Astaurov, 1936, 1937), when as a result of fusion of two haploid sperm nuclei the formation of diploid individuals was observed (Fig. 1). Moreover, when a female was inseminated by two genetically different males, the two-father's hybrids were sometimes obtained (Strunnikov, 1958; Astaurov, 1968).
At last, only quite recently, the cases of androgenesis were found in nature, and then properly explained. The freshwater clam Corbicula sandai (Mollusca: Bivalvia, Corbiculidae) is a common diploid bisexual dioecious species from Lake Biwa, Japan. This species is supposed to breed in a usual way as it has normal reductional haploid spermatozoa possessing half the DNA found in somatic cells. The other two species of the genus, C. leana and C. fluminea, closely related to C. sandai are hermaphrodites (Miyazaki, 1936; Kraemer & Galloway, 1986). It has been shown, that being individually isolated these molluscs are able to produce progenies, that was checked for two generations (Ikematsu & Yamane, 1977). Thus reproduction by self-fertilization was primarily suggested. Later on the gynogenetic development was supposed (Okamato & Arimoto, 1986).
Recently it has been realised that one of the Japanese populations of C. leana is triploid. In Taiwan the individuals of C. fluminea from the same locality appeared to be diploid and triploid (Komaru & Konishi, 1999). It should be also pointed out that triploid as well as diploid molluscs of the genus Corbicula from several examined localities of the Eastern Asia produce non-reductional spermatozoa containing the amount of DNA equal to that of somatic cells (Okamato & Arimoto, 1986; Komaru et al., 1997; Komaru & Konishi, 1999).
Finally, the detailed cytological observations (Fig. 2) and DNA microfluorometry of C. leana made it possible to demonstrate that the developing embryo contains only those chromosomes which were delivered by spermatozoid, while the products of the egg nucleus division are transported to the cell surface and then moved out of it (Komaru et al., 1998). So, it turned out to be quite difficult to distinguish the androgenetic development from parthenogenesis.
Not long ago, the androgenesis was found in the stick-insects (Insecta, Phasmatoptera). In northwestern Sicily the all-female unisexual populations produced as a result of interbreeding of two species (Bacillus rossius and B. grandii benazzii) and possessing one haploid chromosome set of each one were discovered. They and the parental species are sympatric. One of them - B. rossius is bisexual species, females 2n=36, XX, males 2n=35, XO, which includes facultatively parthenogenetic demes. The other one - B. grandii is strictly bisexual, females 2n=34, XX, males 2n=33, XO, and includes three subspecies (Mantovani et al., 1991; Scali, 1991).
In laboratory adult females (B. rossius-grandii benazzii) were mated to males which belong to B. grandii benazzii, B. grandii grandii, B. grandii maretimi, and B. rossius. The eggs laid before mating never hatched, that proves the lack of parthenogenesis. Allozyme analysis of parents and their offspring was carried out. Maternally and paternally inherited genes were revealed by markers (Mantovani & Scali, 1992). From the allozyme analysis of the offspring, three pheno-genotypic classes can be distinguished.

Fig. 1. Interspecies dispermic androgenesis in silkworm (after Astaurov, 1968 simplified)

Fig. 2. Androgenetic development of egg in Corbicula leana (schematised after Komaru, Kawagishi & Konishi, 1998). Maternal chromatin is extruded as two polar bodies. Paternal diploid chromosome set constitutes the metaphase of the first cleavage division. (Insert shows sperm nucleus one minute after its penetration into egg).
The largest class of descendants originated from all crosses is represented by individuals inheriting one entire haploid chromosome set from mother and the other one derived from the fathering male. This is an example of the semi-clonal inheritance that is very common to hybridogenetic breeding in frogs and fishes occurring with the participation of males belonging to closely related species.
The second class includes descendants of both sexes showing only paternal genes. These are clearly androgenetic descendants. Cytological analysis fully supports their all-paternal constitution: the crossed forms differ from each other in number and morphology of chromosomes.
The third class is very rare (only two females were found); it includes the offspring that inherited only maternal genes. One of these females was completely homozygous as far as it was possible to judge from the analysis of few genes; in its genotype one of the maternal haploid set was simply doubled. Another female completely repeats the maternal genotype in its all three loci studied. As wrote Mantovani and Scali (1992: p. 788) "these females must derive from different gynogenetic mechanisms".
It could be added that flexible and being unstable in hybrid forms the mechanisms of the genotype rearrangements which proceed during the meiosis allow the different ways of preservation or restoring of somatic chromosome number in an egg. In the majority of animals meiosis in egg is far from its completion by the time when spermatozoid penetrates into it. Thus, the egg being ready to develop still contains the whole maternal chromosome set (see Vasetsky, 1977). As it has been observed in the stick-insects and many other organisms, when male pronucleus does not participate in development, it can lead to pure maternal inheritance. On the other hand, if the progeny develops without female pronucleus the paternal cloning by use of only chromosomes introduced by spermatozoid is possible (androgenesis in silk-worms, molluscs and stick-insects). Finally, the fusion of maternal pronucleus and one of the polar bodies formed in outcome of meiosis is quite analogous to self-fertilization and leads to either complete or partial homozygosity of the offspring.
In order to differentiate between pure maternal and paternal inheritance which depends on the mechanisms of clonal reproduction, one should use some genetic markers or observe the process of meiosis in detail. However, it is not an easy job. The all-female reptile species known are considered to be parthenogenetic because within the group neither gynogenesis nor any other ways of unisexual reproduction have been ever registered. At the same time in fishes, the unisexual forms are believed to be either gyno- or hybridogenetic, since in all the cases studied the successful development of eggs occurs only after their contact with sperm. Seeing that our knowledge of the meiotic mechanisms in all-female forms is quite incomplete, it will not be surprising if some of the species known as parthenogenetic appear either gynogenetic ones, or even androgenetic hermaphrodites.
The discussion on the matter presented above can lead to a somewhat skeptical attitude towards mitochondrial DNA analysis that is one of the most advanced and attractive methods applied in phylogenetic studies. A few though well-proven examples of androgenesis show that diploid (or polyploid) genotype can be moved by spermatozoid to an egg of another species. Therefore even the identity of mitochondrial DNA possessed by some forms cannot be considered as indisputable evidence of their close phylogenetic relations. In the history of species nuclear genome can part from mitochondrial one.
Astaurov, B.L. 1936. Artificial parthenogenesis and androgenesis in silk-worm. Byull. vses. Akad. sel'skohoz. Nauk 12: 47-52. (In Russian).
Astaurov, B.L. 1937. Tests on the experimental androgenesis and gynogenesis in silk-worm. Biol. Zh. 6 (1): 1-50. (In Russian).
Astaurov, B.L. 1968. Tsytogenetika razvitiya shelkovichnogo chervya i ego eksperimental'nyi kontrol [Cytogenetics of the development of silk-worm and its experimental control]. Moskva, Nauka. 102 pp. (In Russian).
Berg, L.S. 1947. On the "unisexual" breeding in crucian carp. Vest. Leningr. gos. Univ. (7): 55-59. (In Russian).
Berger, L. 1967. Embryonic and larval development of F1 generation of green frogs different combinations. Acta zool. cracov. 12 (7): 123-160.
Borkin, L.J. & I.S. Darevsky. 1980. Reticulate (hybridogenetic) speciation in vertebrates. Zh. obshch. Biol. 41 (4): 485-506. (In Russian).
Borkin, L.J., Vinogradov, A.E., Rosanov, J.M. & A.I. Caune. 1987. Semiclonal population in Rana esculenta hybridogenetic complex: evidence from DNA cytometry. Dokl. Akad. Nauk SSSR 295 (5): 1261-1264. (In Russian).
Cherfas, N.B. 1987. Gynogenesis in fishes. In: Kirpichnikov V. S. Genetika i selektsiya ryb [Genetics and selection of fishes]. 2nd edn. pp. 309-335. Leningrad, Nauka. (In Russian).
Cuellar, O. 1974. The origin of parthenogenesis in vertebrates: the cytogenetic factors. Am. Nat. 108: 625-648.
Echelle, A.A. & D.T. Mosier. 1981. All-female fish: a cryptic species of Menidia (Atherinidae). Science 212 (4501): 1411-1413.
Echelle, A.A. & D.T. Mosier. 1982. Menidia clarkhubbsi n. sp. (Pisces: Atherinidae) an all-female species. Copeia (3): 533-540.
Echelle, A.A., Echelle, A.F. & C.D. Crozier. 1983. Evolution of an all-female fish, Menidia clarkhubbsi (Atherinidae). Evolution 37 (4): 772-784.
Golovinskaja, K.A. & D.D. Romashov. 1947. Investigation on the gynogenesis of crucian carp. Trudy vses. nauchno-issled. Inst. prud. rybn. Khoz. 4: 73-113. (In Russian.)
Grant, V. 1977. Organismic evolution. San Francisco.
Hasimoto, H. 1934. Formation of an individual by the union of two sperm nuclei in the silkworm. Bull. Seric. exp. Stn Jap. 8 (10): 455-464.
Hubbs, C.L. & L.C. Hubbs. 1932. Apparent parthenogenesis in nature in a form of fish of hybrid origin. Science 76 (1983): 628-630.
Ikematsu, W. & S. Yamane. 1977. Ecological studies of Corbicula leana Prime. III. Bull. Jap. Soc. scient. Fish. 43: 1139-1146.
Komaru, A., Kawagishi, T. & K. Konishi. 1998. Cytological evidence of spontaneous androgenesis in the freshwater clam Corbicula leana Prime. Devl. Genes. Evol. 208: 46-50.
Komaru, A. & K. Konishi. 1999. Non-reductional spermatozoa in three shell colore types of the freshwater clam Corbicula fluminea in Taiwan. Zool. Science 16: 105-108.
Komaru, A., Konishi, K., Nakayama, I., Kobayashi, T., Sakai, H. & K. Kawamura. 1997. Hermaphroditic freshwater clams in the genus Corbicula produce non-reductional spermatozoa with somatic DNA content. Biol. Bull. 193: 320-323.
Kraemer, L.R. & M.L. Galloway. 1986. Larval development of Corbicula fluminea (Müller): An appraisal of its heterochrony. Am. malac. Bull. 4: 61-79.
Lada, G.A., Borkin, L.J. & A.E. Vinogradov. 1995. Distribution, population systems and reproductive behavior of green frogs (hybridogenetic Rana esculenta complex) in the Central Chernozem Territory of Russia. Russ. J. Herpetology 2 (1): 46-57.
MacGregor, H.C. & Th.M. Uzzell (Jr.). 1964. Gynogenesis in salamanders related to Ambystoma jeffersonianum. Science 143 (3610): 1043-1045.
Mantovani, B., Scali, V. & F. Tinti. 1991. Allozyme analysis and phyletic relationships of two new stick-insects from north-west Sicily: Bacillus grandii benazzii and B. rossius-grandii benazzii (Insecta Phasmatodea). J. evol. Biol. 4: 279-290.
Mantovani, B. & V. Scali. 1992. Hybridogenesis and androgenesis in the stick insect Bacilius rossius-grandii benazzii (Insecta, Phasmatodea). Evolution 46 (3): 783-796.
Maynard Smith, J. 1971. The origin and maintenance of sex. In: Group selection. (G.C. Williams. Ed.). pp. 163-175. Chicago, Aldine-Atherton.
Maynard Smith, J. 1978. The evolution of sex. Cambridge, Cambridge University Press. 271 pp.
Miyazaki, I. 1936. On the development of bivalves belonging to the genus Corbicula. Bull. Jap. Soc. scient. Fish. 5: 249-254. (In Japanese, with English abstract.)
Okamato, A. & B. Arimoto. 1986. Chromosomes of Corbicula japonica, C. sandai and C. leana (Bivalvia: Corbiculidae). Venus 45: 194-202.
Sanderson, A.R. 1960. The cytology of a diploid bisexual spider beetle Ptinus clavipes Panzer and its triploid gynogenetic form mobilis Moor. Proc. R. Soc. Edinb. (B) 67: 333-350.
Scali, V. 1991. Un nuovo insetto stecco (Phasmatodea) della Sicilia: Bacillus grandii benazzii (n. subsp.). Frustula ent. (n. ser.) 12 (1989): 397-408.
Schultz, R.J. 1967. Gynogenesis and triploidy in the viviparous fish Poeciliopsis. Nature 157: 1564-1567.
Schultz, R.J. 1969. Hybridization, unisexuality, and polyploidy in the teleost Poeciliopsis (Poeciliidae) and other vertebrates. Am. Nat. 108: 605-619.
Schultz, R.J. 1977. Evolution and ecology in unisexual fishes. In: Evolutionary biology. (M. Hecht, W. Steere and B. Wallace. Eds.). Vol. 10. pp. 277-331. New York, Plenum Press.
Strunnikov, V.A. 1958. Breeding of double-paternal androgenetic hybrids in silk worm. Dokl. Akad. Nauk SSSR 122 (3): 516-519. (In Russian.)
Suomalainen, E. 1969. Evolution of parthenogenetic Curculionidae. In: Evolutionary biology. (T. Dobzhansky et al. Eds.). Vol. 3. pp. 261-296. New York, Plenum Press.
Suomalainen, E. & A. Saura. 1973. Genetic polymorphism and evolution parthenogenetic animals. I. Polyploid Curculionidae. Genetics 74: 489-508.
Suomalainen, E., Saura, A. & J. Lokki. 1976. Evolution of parthenogenetic insects. In: Evolutionary biology. (M. Hecht et al. Eds.). Vol. 9. pp. 209-257. New York, Plenum Press.
Tinti, A. & V. Scali. 1992. Genome exclusion and gametic DAPI-DNA content in the hybridogenetic Bacillus rossius-grandii benazzi complex (Insecta, Phasmatodea). Molec. Reprod. Devl. 33: 235-242.
Tinti, A. & V. Scali. 1996. Androgenesis and triploid from interacting parthenogenetic hybrid and its ancestors in stick insect. Evolution 50: 1251-1258.
Vasetsky, S.G. 1977. Meiotic divisions. In: Sovremennye problemy oogeneza [Modern problems of oogenesis]. pp. 145-171. Moskva, Nauka. (In Russian).
Vasil'ev, V.P. 1985. Evolyutsionnaya kariologiya ryb [Evolutionary karyology of fishes]. Moskva, Nauka. 300 pp. (In Russian).
Vasil'ev, V.P. & E.D. Vasil'eva. 1982. New diploid-polyploid complex in fishes. Dokl. Akad. Nauk SSSR 266 (1): 250-252. (In Russian).
Vinogradov, A.E., Borkin, L.J., Günter, R. & J.M. Rosanov. 1990. Genome elimination in diploid and triploid Rana esculenta males: cytological evidence from DNA flow cytometry. Genome 33: 619-627.
Vrijenhoek, R.C. 1972. Genetic relationships of unisexual hybrid fishes to their progenitors using lactate dehydrogenase isozymes as gene markers (Poeciliopsis, Poeciliidae). Am. Nat. 106 (952): 754-766.