© 2000, Annual Reports of the Zoological Institute RAS.
Elena M. Chaban
Zoological Institute, Russian Academy of Sciences, Universitetskaya nab., 1, St. Petersburg, 199034, Russia
The family Retusidae Thiele, 1925 includes some of the smallest opisthobranchs of the order Cephalaspidea without radula. Shells of retusids like other families of cephalaspideans are not characterized by great morphological diversity: sculpture is absent or formed by longitudinal striae, fluted ribs, lines of growth, sometimes equipped with spiral striae. Spire from elevated to sunken. Aperture is simple without any projections and folds. Descriptions of genera are based on shell and gizzard plates morphology, but descriptions of species in the majority of cases include shell morphology only. That is why even though more than fifty species of Retusa Brown, were described in the last two centuries and in spite of species of Retusa being a usual component of ecosystems of sandy-mud substrate, faunal and taxonomic papers on well studied North Atlantic habitats include as a rule only three species of Retusa according to the degree of protruding of the spire: R. obtusa (Montagu), R. truncatula (Bruguiere) and R. umbilicata (Montagu) (Lemche, 1948; Thompson, 1976). Recent works on fauna of Russian seas also include three species of Retusa showing differences in shell morphology. R. pertenuis (Mighels) has an oval shell with slightly elevated spire, and is distributed in the Arctic seas (Golikov, 1995). R. instabilis Minichev has a cylindrical shell with a slightly elevated or truncated spire and not very strongly developed longitudinal sculpture, and was described from the north-eastern part of the Japan Sea (Minichev, 1971). R.(Cylichnina) succincta (A. Adams) from the Japan Sea (Minichev, 1971) has a shell with sunken spire. The real morphological diversity is much higher however. Therefore we need to differentiate the species according to some other morphological characteristic in addition to the shell. For this purpose we can use the morphology of male copulatory system like in other families of cephalaspideans. Firstly the morphology of the genital system of Retusa was studied by Minichev (1967) for R. operculata Minichev and simultaneously by Smith (1967) for R. obtusa. Later this characteristic was studied for other species: R. truncatula (Mikkelsen, 1995), R. pelix Burn, and R. chrysoma (Burn & Bell, 1974a, 1974b). In other genera of Retusidae the male genitalia were studied for Relichna murdochi (Suter) by Rudman (1971). Retusa operculata has a male copulatory complex equipped with three prostates and Minichev included this feature in the description of the genus Retusa as a generic character (Minichev, 1967). The species of Retusa have differences in the male copulatory system, however. The goal of the present work was to study the structure of the male copulatory complex of retusids of the Russian seas.
More than a thousand specimens of Retusa and Volvulella Newton kept in collections of the Laboratory of Marine Research of the Zoological Institute, Russian Academy of Sciences were studied. The range of the collected specimens includes the North Atlantic, the Arctic, the Russian Pacific and the South China Sea. About 40 specimens were dissected under binocular microscope and the male copulatory complex and gizzard plates were studied and drawn with a camera lucida. The parts of male copulatory system include penial, subpenial and accessory prostates according to Berry et al. (1992) or prostates and ejaculatory duct according to Rudman (1971).
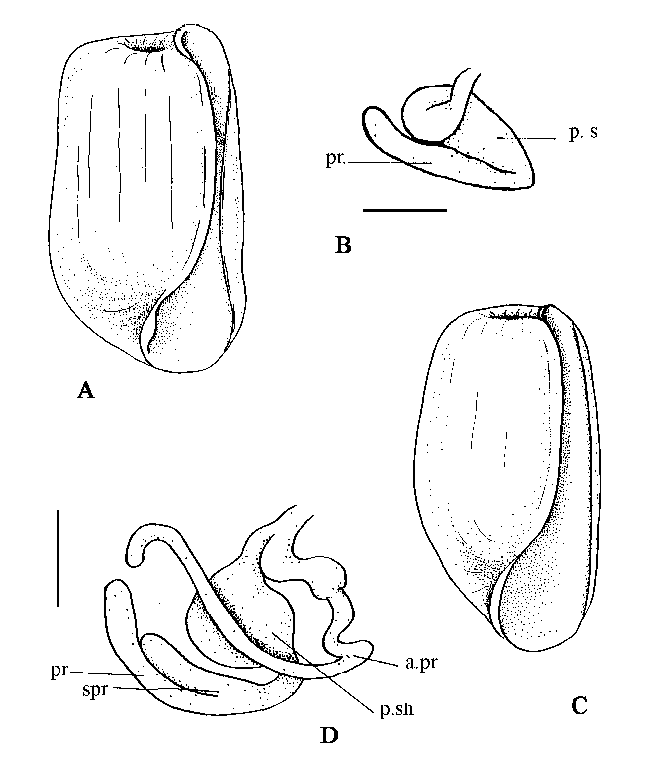
Our investigations showed a high diversity of the male genitalia system in the family of Retusidae. Retusa sp. n. 1 (Bering Sea, Vrangel), R. sp. n. 2 (Laptev Sea) and R. semen (Reeve) (Kara Sea) like R. obtusa from the North Atlantic have four prostates: penial, subpenial and two accessory ones. The two accessory prostates of R. obtusa and R. semen connected with the penial sac but one of accessory prostates of R. sp. n.1 and R. sp. n. 2 connected with the internal ciliated duct in the proximal part of the male copulatory complex. R. pertenuis (the Arctic and Japan Sea), R. turrita (Moeller) (White, Barents and Kara seas), R. succincta (Fig. 1, C-D) and R. toyamaensis (Habe) (Japan Sea) have three prostates: penial, subpenial and one accessory prostate. R. truncatula, R. umbilicata (North Atlantic), R. minima Yamakawa (Fig. 1, A-B), R. truncata Yamakawa 1911 (=R. instabilis Minichev 1971 - syn. nov.) (Japan Sea) have one penial prostate and sometimes a small additional extension (subpenial prostate?) of the penial sac needed for histological study. This diversity of the male genitalia does not correlate with the morphology of the gizzard plate structure and cannot be used to distinguish genera but can be used as a good characteristic for the defining of species.

Fig. 1. Shells and male copulatory system of Retusidae. Retusa minima (A-B): A - shell, ventral view, h - 2.1 mm; B - male copulatory system. Japan Sea, Posjet Bay, st. 56/10, 11 m, mud, clay, 10.07.1962, coll. Mikulich. Retusa succincta (C-D): C - shell, ventral view, h - 3.2 mm; D male copulatory system. Japan sea, Peter the Great bay, st. 17, 65 m, sand, 15.08.1970, coll. Klimova. Abbreviations: apr - accessory prostate, pr - penial prostate, psh - penial sheath, spr - subpenial prostate. Scale bar: B - 0.2 mm, D - 0.4 mm
In some cases we have ecological data, which confirm reality of the species, distinguished on the differences of the male copulatory system. For example R. obtusa inhabits shallow waters and estuaries. It feeds not only on foraminiferans but also on gastropods like Hydrobia ulvae (Smith, 1967). The shell of R. obtusa has a characteristic expansion of the lower part of the mouth. But R. pertenuis which is known as a synonym of R. obtusa (Lemche, 1948), inhabits a depth range from 7 to 515 m with salinity between 33-34‰. R. truncata and R. sp .n. 2 inhabit shallow waters like R. obtusa but they have a different shell and genital morphology.
The form of the shell and the gizzard plates was used by Thiele (1925) as generic and subgeneric characteristics as well as by other authors of recent check-lists (Vaught, 1988; Millard, 1997). I think that we cannot use the form of the shell as a generic and subgeneric characteristic because intermediate forms exist between species with absolutely sunken spire (R. umbilicata), species with flat spire shells (R. truncata), and species with shells with elevated spire (R. longispirata Yamakawa). For the last form generic or subgeneric division is absent. Some authors include forms with elevated spire inside the genus Decorifer Iredale (Higo & Goto, 1993) belonging this genus to Retusidae. But the genus was established for species (D. elisae Iredale) with a flat spire and unknown internal morphology. The systematic position of Decorifer is uncertain and we cannot use it for other species of Retusidae. Sometimes this genus is considered to belong to Acteocinidae (Lin Guang-yu & Qi Zhong-yan, 1985).
It is interesting that, as noted by Pilsbry (1895), Bulla truncatula Bruguiere is the type species of Retusa Brown as well as of Coleophysis Fischer and the latter is a junior synonym of the former. Despite this sometimes Coleophysis is used as a valid subgenus of Retusa or even as a genus (Zilch, 1960; Higo & Goto, 1993; Millard, 1996).
At present time gizzard plates morphology only may be used for separating genera. Pyrunculus Pilsbry and Relichna Rudman are well defined genera of the family Retusidae. The first has gizzard plates with two teeth (Thiele, 1925) and gizzard plates of the second are equipped with closely packed chitinous rods forming concave inner surface (Rudman, 1971). Retusa has gizzard plates equipped with numerous protuberances, and the shell form varying very widely. Retusa toyamaensis (Habe) has a slightly pear-shaped form of the shell as Pyrunculus, but its plates are equipped with numerous protuberances and it really belongs to Retusa (Chaban, 1999) as well as Retusa tokyoensis (Habe) - comb. nov. Contrary to these examples, Tringali and Oliverio (1999) have studied Pyrunculus fourierii (Audouin) and found that despite its Retusa-like shell morphology (the species is in fact usually included in Retusa) the gizzard plates are typical of Pyrunculus.
Within cephalaspideans collected by O.A. Scarlato during the expedition of the Zoological Institute to the South China Sea some specimens were identified as Semiretusa borneensis (A. Adams) by A.V. Martynov (personal communication) who has found radula in it. That is why taxon Semiretusa Thiele must be excluded from Retusidae.
Volvulella is placed into the family Retusidae because of the absence of radula. It is distinguished from another genera of the family by the absence of gizzard plates. But the reduction of gizzard plates and radula in Volvulella corresponds to the transformation of its hole alimentary system. It consists of tubular pharynx and esophagus without crop or gizzard. There is a tubular 'proboscis' inside of the pharynx. There are no tracks of foraminiferans or visible fractions inside its alimentary canal. It may be possible that 'proboscis' can project from the pharynx and can be used for feeding may be on detritus. The taxonomy of Cephalaspidea is based on alimentary canal characteristics. Differences in morphology of this system in Volvulella on the one hand and in Retusa on the other hand are needed for differentiating family for Volvulella. This family was established and named Volvulidae Locard 1892 and later Rhizoridae Dell 1956. But the nominative genus for Volvulidae - Volvula A. Adams 1850 - was preoccupied by Volvula Gistel 1848 (Insecta, Diptera) and the nominative genus for Rhizoridae - Rhizorus Montfort 1810 cannot be senior synonym of Volvulella because many differences exist between their type species, Rhizorus adelaidis Montfort and Volvulella acuminata (Bruguiere) (Harry, 1967). That is why I propose a new family name: Volvulellidae nom. nov. pro Volvulidae Locard.
I would like to thank Mr. Marc Lang for correcting English.
Berry, A.J., Purvis, J. & K.V. Redhakrishnan. 1992. Reproductive system and spermatogenesis in the opisthobranch gastropod Retusa obtusa (Montagu). J. mollusc. Stud. 58: 357-367.
Burn, R. & K.N. Bell. 1974a. Description of Retusa pelyx Burn sp. nov. (Opisthobranchia) and its food resources from Swan Bay, Victoria. J. malac. Soc. Aust. 3 (1): 37-42.
Burn, R. & K.N. Bell. 1974b. Description of Retusa chrysoma Burn sp. nov. (Opisthobranchia) and its food resources from Corner inlet, Victoria. Mem. natn. Mus. Vict. 35: 115-119.
Chaban, E.M. 1999. Morphology of male reproductive system in taxonomy of the genus Retusa Brown, 1827 (Cephalaspidea: Retusidae). In: Systematic, Phylogeny and Biology of Opisthobranch Molluscs: 2nd International Workshop of Malacology, Menfi, June 10-14 1999. Abstracts. p. 26. Menfi.
Golikov, A.N. 1995. Shell-bearing gastropod molluscs of the Arctic Ocean. Moscow, Tropa. 185 pp.
Higo, Sh. & Y. Goto. 1993. A systematic list of molluscan shells from the Japanese Is. and adjacent area. Elle Scientific Publ. 148 pp.
Harry, H.W. 1967. A review of the living tectibtanch snails of the genus Volvulella with descriptions of a new subgenus and species from Texas. Veliger 10: 133-147.
Lemche, H. 1948. Northern and Arctic tectibranch gastropods. Biol. Skr. 5 (3): 1-136.
Lin Guangyu & Qi Zhong-yan. 1985. A preliminary survey of the Cephalaspidea (Opisthobranchia) of Hong Kong and adjacent waters. In: Proceedings of the 2nd International Workshop on the Malacofauna of Hong Kong an Southern China, Hong Kong, 1983. (B. Morton and D. Dudgeon. Eds.). pp. 109-124. Hong Kong University Press.
Minichev, Y.S. 1967. Studies on the morphology of the lower Opisthobranchia (on the evolutionary significance of the detorsion process). Trudy Zool. Inst., Leningrad 44: 109-182. (In Russian).
Minichev, Y.S. 1971. On fauna, ecology and taxonomy of Retusidae (Opisthobranchia Cephalaspidea) of the Possyet Bay of the Japan sea. In: Issledovaniya fauny morey [Studies of marine faunas]. Vol. 8 (16). pp. 3-322. Leningrad, Nauka. (In Russian).
Mikkelsen, P.M. 1995. Cephalaspid opisthobranchs of the Azores. Açoreana suppl.: 193-215.
Millard, V. 1997. Classification of Mollusca. A classification of world wide Mollusca. Pretoria, South Africa. 544 pp.
Pilsbry, H.A. 1893. Manual of conchology. Structural and systematic. Vol. 15. Philadelphia, Conchological Section Publ. 436 pp.
Rudman, W.B. 1971. On a new genus for "Tornatina" murdochi Suter, 1913 (Retusidae, Opisthobranchia). J. malac. Soc. Aust. 2 (2): 187-193.
Smith, S.T. 1967. The development of Retusa obtusa (Montagu) (Gastropoda, Opisthobranchia). Can. J. Zool. 45: 737-764.
Thiele, J. 1925. Gastropoda der Deutschen Tiefsee Expedition. 2: Wissenschaftiliche ergebnisse der Deutschen Tiefsee-Expedition auf dem Dampfer "Valdivia" 1898-1899. Bd. 17 (2). S. 4-382. Jena, Gustav Fisher Verl.
Thompson, T.E. 1976. Biology of opisthobranch molluscs. Vol. 1. London, The Ray Society Publ. 207 pp.
Tringali, P. & M. Oliverio. 1999. The Mediterranean species of the genus Pyrunculus Pilsbry, 1895 (Cephalaspidea, Retusidae). In: Systematic, phylogeny and biology of opisthobranch molluscs: 2nd International Workshop of Malacology, Menfi, June 10-14 1999. Abstracts. p. 33. Menfi.
Vaught, K.C. 1989. A classification of the living Mollusca. (R.T. Abbott and K.J. Boss. Eds.). Melbourne, Florida, American Malacologists Inc. 195 pp.
Zilch, A. 1960. Gastropoda: Euthyneura. In: Handbuch der Paläozoologie. Bd. 6 (2). (W. Wenz. Ed.). Berlin, Borntraeger. 834 S.